Manipulating reactivity of black phosphorous using protective chemistry
Scientists can regulate the reactivity of molecules and functional groups in commercial and lab-based synthetic organic chemistry processes. The idea can be implemented in inorganic nanomaterials, including 2D black phosphorus (BP) nanosheets. For instance, when the compound is not being used, the chemists can “shut down” the high reactivity of a few or monolayer BP to continue its activity upon utilization. A protective chemistry-based approach to regulate the reactivity of black phosphorus is developed by Xiao Liu and a group of researchers in physics, biomaterials, biological chemistry, and chemical engineering in China. The outcomes of the study are released in Science Advances.
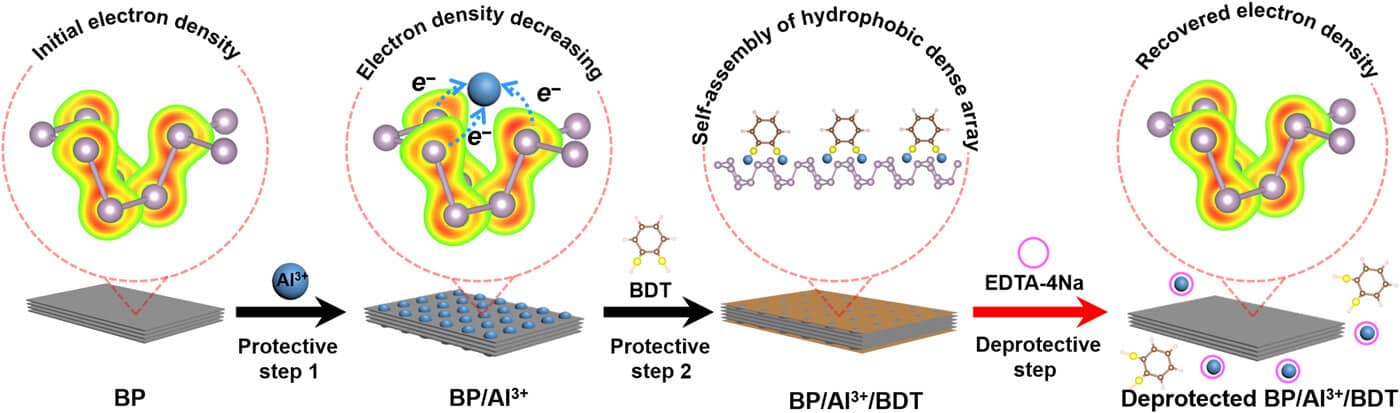
The team bound aluminum cations ( Al3+) with lone pairs of electrons from black phosphorous and reduced the electron density on the BP surface to start the protective step. Using an oxygen/water-resistant layer via the self-assembly of hydrophobic 1,2-benzenedithiol (BDT) on the BP/Al3+ conjugate, the team finished the process. A stable compound with reduced reactivity was formed from the protective step. To achieve the deprotection of the BP/Al3+/ BDT complex to eliminate Al3+ and BDT from the BP surface, the team used a chelator treatment process. Using the deprotection step to restore reactivity to the compound, the team recovered the electron density of BP.
Tuning the properties of nanomaterials
In nanoscience, scientists can specifically tune the characteristics of nanomaterials to acquire the required features. For multistep programmable uses, controlling the reactivity of nanomaterials is vital. Few nanomaterials can be protected to minimize their reactivity under particular conditions and recover activity after effective deprotection. For this reason, scientists have recommended a series of particular and reliable approaches to control the reactivity of functional groups in organic chemistry.
Though, the present established organic protective-deprotection processes can not be used in inorganic nanomaterials because of the lack of similar functional groups. Thus, a reliable and basic method to regulate the reactivity of inorganic materials prevails to be established. To complete this, the group began binding BP with Al3+ to reduce the surface electron density and efficiently reduce its reactivity. To provide an ultra-stable complex (BP/ Al3+ / BDT), the protective process created an array of the hydrophobic 1,2-benezendithiol molecule in the presence of BP and aluminum cations. Up to 2 months, the stability of the compound was maintained under ambient environments, without modifications.
Synthesizing and characterizing BP/Al3+/ BDT compound
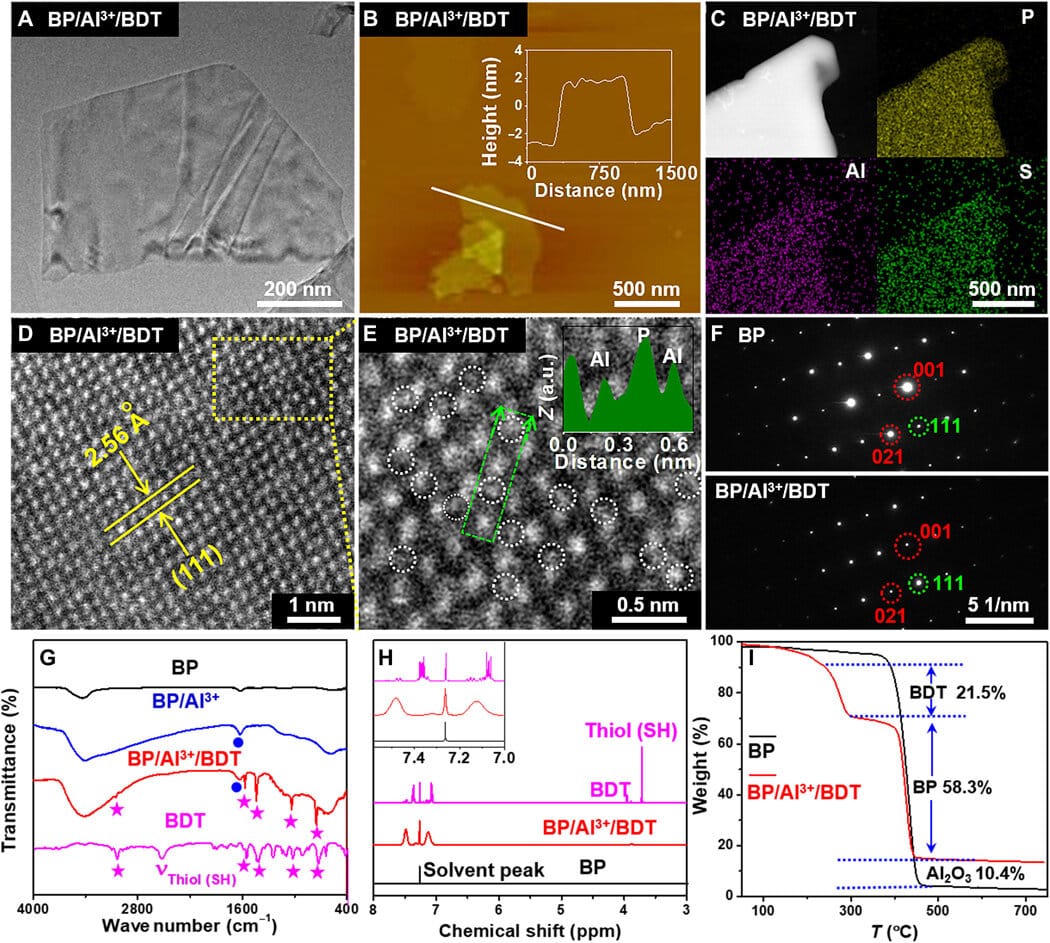
The study group manufactured and characterized (tested) the mass BP by using a formerly known procedure. They initially got the BP nanosheets by sonicating the powdered form of the molecule; after that, they analyzed the dimensions of BP utilizing SEM and TEM. An insight into the nanosheet structure was provided by high-resolution TEM. Atomic force microscopy disclosed the density of BP with 4-6 specific layers of phosphorene. They ascertained the crystal characteristics of BP to be comparable to its bulk type, utilizing X-ray diffraction and Raman spectra. They blended BP with lightweight aluminum chloride in an ethanol solution during the protective step of cationic binding to the BP surface.
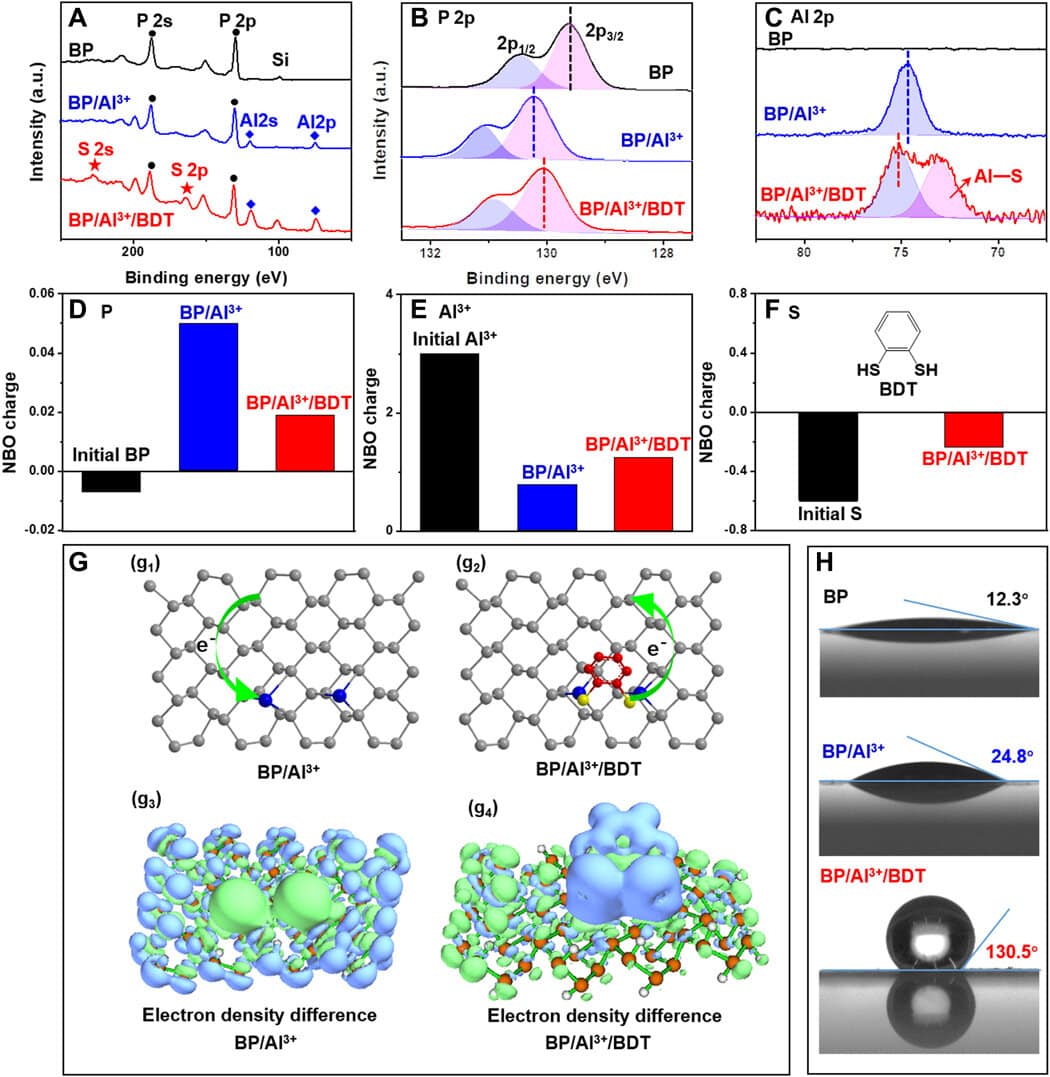
Later, by using X-ray photoelectron spectroscopy, the team identified the successful attachment of aluminum cations to the black phosphorus surface to enhance the protection of black phosphorus. Utilizing scanning transmission electron microscopy, they examined the nano-morphology of the compound and analyzed the surface conformation, and additionally verified its framework using FTIR and 1H NMR spectroscopy.
Manipulating reactivity of black phosphorous/aluminum cations/hydrophobic 1,2-benzenedithiol
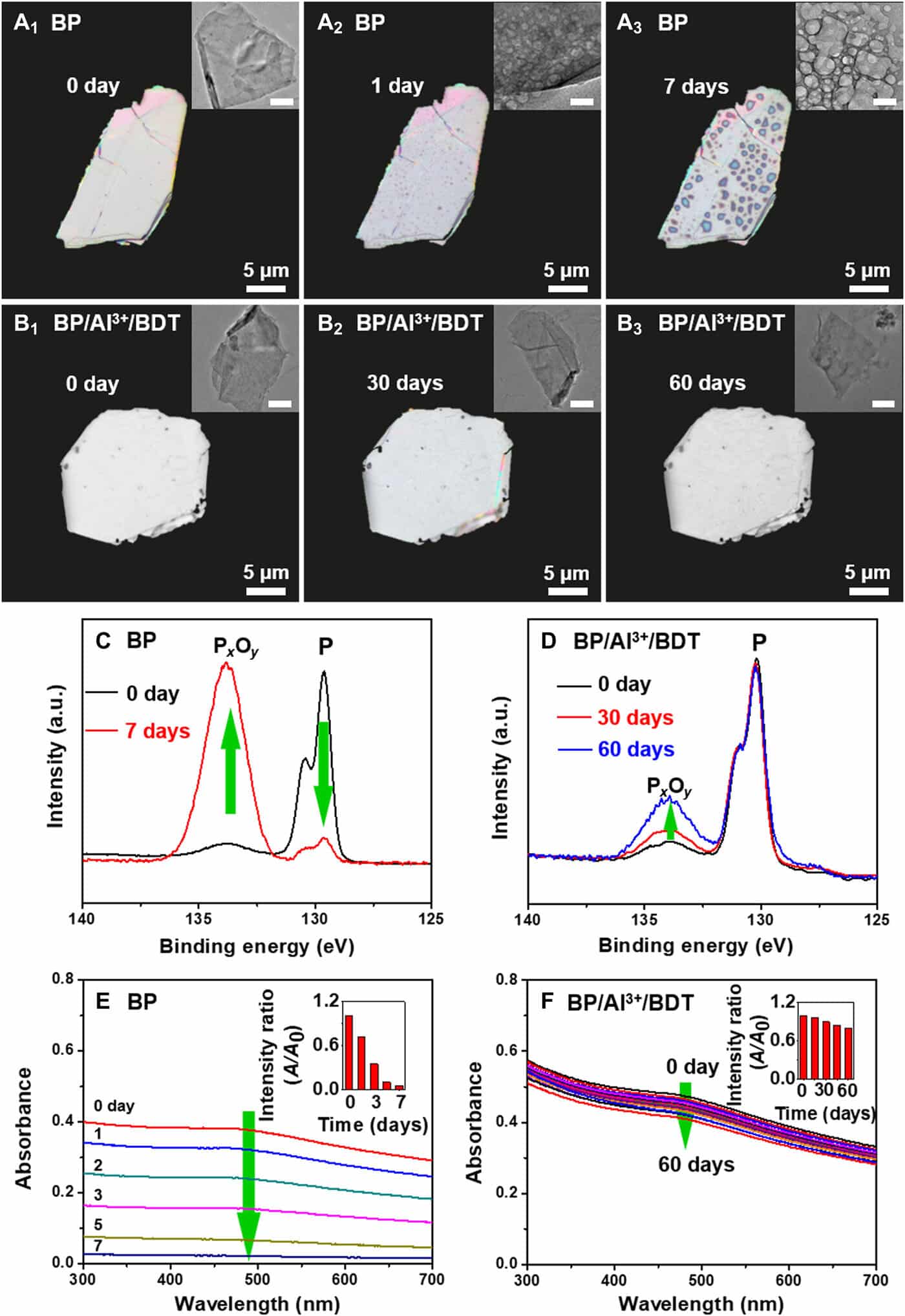
The team examined the reactivity of the black phosphorus/aluminum cations/hydrophobic 1,2-benzenedithiol complex by using a range of optical methods in material science to comprehend its framework. The evolution of the crystal structure and long-lasting stability under ambient environments was revealed by the analogous images. The stability of the BP/Al3+/BDT complex was better than BP alone, and the outcomes featured the usefulness of embedding BP in the compound. The researchers attributed the decreased reactivity of the complex structure to 2 elements – the Al3+ binding the BP surface contributed to reducing chemical reactivity, and the self-assembled hydrophobic dense array of BP surface separated the molecule from oxygen and water to stop more destruction, increasing the stability of the compound.
Deprotection of the black phosphorus/aluminum cations/hydrophobic 1,2-benzenedithiol
The study group removed the Al3+ from the compound surface to deprotect the ultra-stable complex. They completed this step with a traditional steel ion chelator – EDTA-4Na. The team additionally eliminated the hydrophobic BDT molecules along with Al3+ to acquire the resulting hydrophilic surface with negative zeta potential, alike in the form of the original BP molecule. The protective-deprotection regulation method allowed the researchers to reversibly control the reactivity of BP. The outcomes recommend that the capability to successfully control the reactivity of the molecule in use.
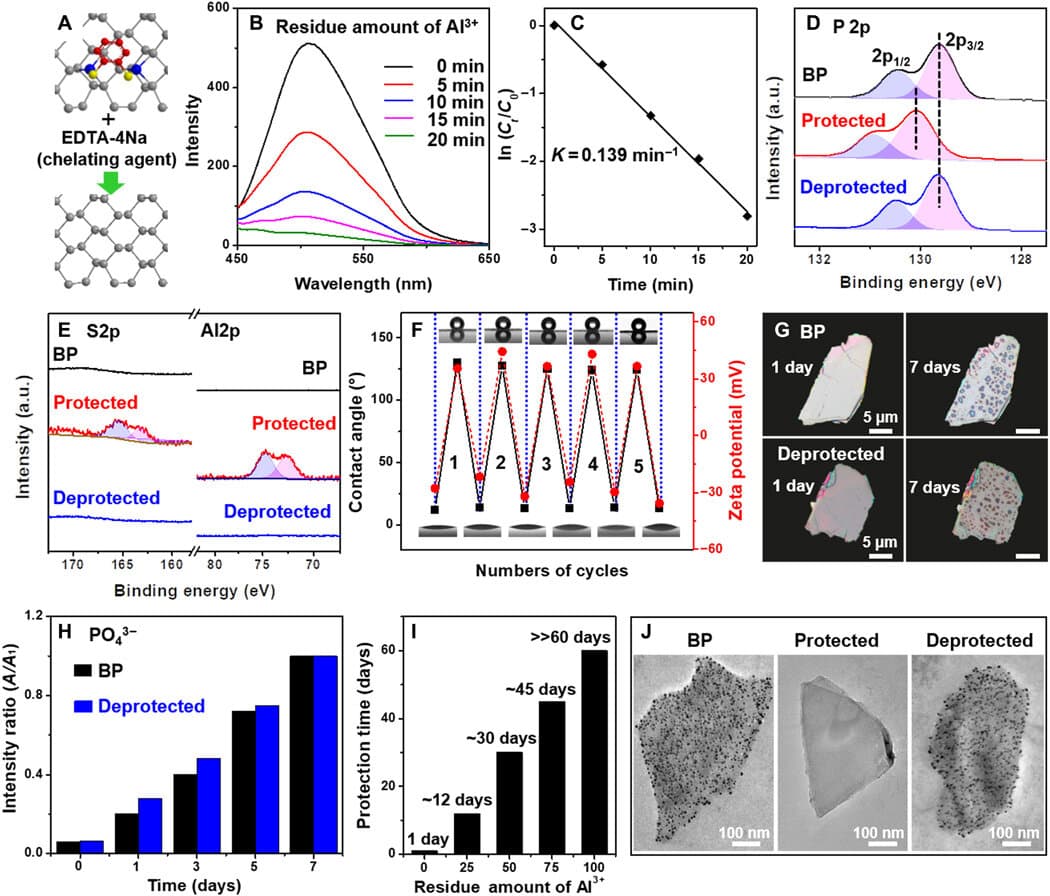
The approach offered a tunable strategy to control the reactivity of BP, which is difficult to accomplish via standard functionalization. This method can be examined to control the reactivity of nanomaterials to develop innovative programmable nanostructures for use in material science.
Manipulating reactivity of black phosphorous















































