2D polymer discovery
Scientists from the U.S. Army Combat Capabilities Development Command, now called DEVCOM, Army Research Lab, collaborated with Professor Steve Lustig, Northeastern University, to speed up the growth of 2D polymers for army uses.

This partnership has led to groundbreaking 2D polymer discovery in the nascent science of 2D polymers. The outcomes of the research are issued in the peer-reviewed scientific journal Macromolecules.
Dr. Eric Wetzel, research area leader for Soldier Materials at the lab, stated that 2D polymers had been researched extremely seriously from a synthetic point of view for just around one decade. They represent a novel, fairly unexplored class of products with great potential.
Wetzel stated that 2D polymers have a really repeatable, symmetrical pattern similar to “chicken wire,” which provides access to more structural improvements contrasted to 1-D, linear polymers like Kevlar.
In an initiative to assess these materials’ complete potential, Army researchers have begun to computationally design 2D polymers in the expectation that they may establish an exceptional choice to traditional aramid fibers for uses like armor and fire-resistant apparel.
Professor Steve Lustig from Northeastern University utilizes his experience with DuPont to help Army researchers determine the environmental
sturdiness of simulated 2D polymers.To correctly build a 2D polymer that can endure actual problems, Army researchers endeavored the aid of Lustig, who earlier worked at DuPont Central Research & Development for more than 20 years before he ended up being an associate professor with tenure at Northeastern University.
Lustig stated that the concept of the 2D polymer project is essential to create a 2D variant of Kevlar. He had more than 10 years of expertise working with the Kevlar business in different liquid crystalline polymerization phases, properties, and processing. The researchers from Army Research Lab believed that his work experience would be advantageous.
He further added that he had initially heard about the lab in the mid-2000s when he came in touch with Dr. Kenneth Strawhecker, an Army scientist who had connected to DuPont in search for sector partnerships.
During this time, Lustig served as a lead scientist in DuPont’s polymer physics group and concentrated on developing new devices for molecular simulations, statistical thermodynamics, and statistical mechanics.
Along with his competence on the computational side of industrial research, he additionally performed tests in polymer processing, atomic pressure microscopy, polymer material property characterization, and chemical synthesis.
Lustig stated that he never had the perseverance to stay in a single area and become a master in just one small field. He has constantly attempted to solve problems holistically utilizing experiments, concepts, and computers.
Once Lustig met with Strawhecker, both started a series of informal partnerships that concentrated on using atomic force microscopy to comprehend Kevlar materials’ structure and their response to bending mechanics and tensile strain.
Lustig proceeded with his collaboration with the lab as a visiting researcher, even after leaving DuPont in 2016. Soon after one of his seminar at the laboratory, he met Wetzel, that promptly realized the importance of Lustig’s industry expertise. Soon after, Lustig joined the Dept of Chemical Engineering, Northeastern University, and became an ARL Joint Faculty Appointment.
According to Strawhecker and Wetzel, Lustig was a leading bidder for the post because of his closeness to ARL’s Northeast campus.
Wetzel stated that the ARL Open Campus Initiative offers a means to penetrate the outside experience that may not be available within their lab.
As per Wetzel, Lustig’s profuse history with high-performance fiber growth projects at DuPont gave Army researchers access to novel modeling abilities along with beneficial support on the processes and techniques that would improve the durability of their conceptual 2D polymers.
Lustig evaluated the environmental durability of the lab’s 2D polymer models and operated computer simulations that ascertained how well they sustain extreme conditions like intense heat.
The molecular simulations of polymers were essential for controlling the computations, which were contributed by Dr. Jan Andzelm, an Army scientist and ARL fellow.
The scientists correlated the thermal stability of a theoretical 2D polymer created by the lab called graphamid, a 2D polymer amide covalent organic framework (amCOF), and 1D polymer Kevlar using these computer simulations.
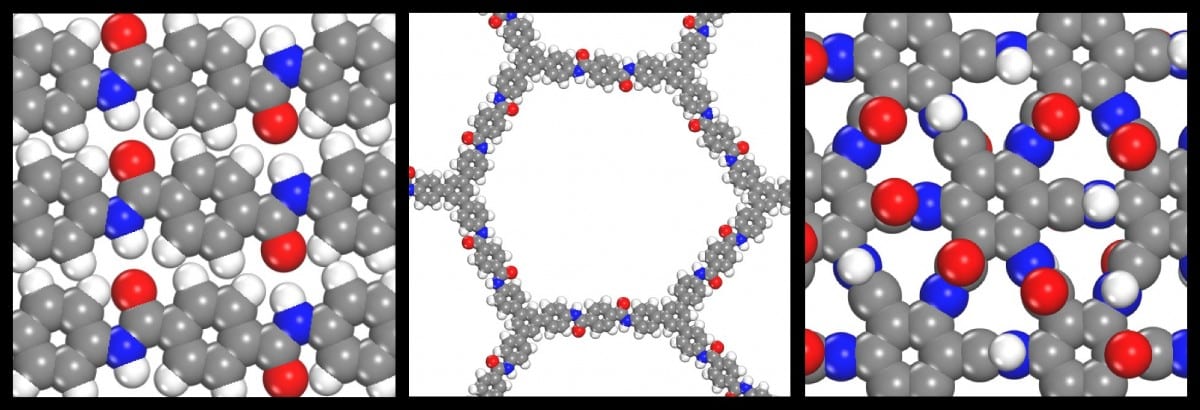
Lustig stated that they executed a series of precise, top-level quantum mechanical computations known as ab initio molecular dynamics and examined the framework’s variations between the 3 molecules they looked at. After they validated that their approach could precisely define a widely known molecule like Kevlar, they could apply it to molecules they did not know, like graphamid, and make precise forecasts about their characteristics and behavior.
The comparison research outcomes revealed that graphamid might potentially endure temperatures as high as 700ºC, which went beyond the limits of both Kevlar and the amide covalent organic framework product.
2D polymer discovery
Also read:
-
WHO Agrees On Compensation Plan In Case Of Severe Adverse Events Of COVAX Vaccine
-
Chemistry & Computer Science Join Forces To Apply Artificial Intelligence To Chemical Reactions

















































