Mechanism Behind Grignard Reaction Uncovered
The Grignard reaction is a crucial step in making new molecules for industrial and academic uses as it is used to synthesize carbon-carbon bonds. Finding selective and efficient methods for this reaction, using minimal energy resources and low-cost materials had been the target of researchers for more than 100 years. The way Grignard reaction works was a mystery until today. Now that the researchers finally understood the reaction, it will open up new improvements.
Carbon is the building block of all living organisms including enzymes, proteins, fats, nucleic acids as well as the essential component of many daily-life materials like plastics, drugs, and fuels. Our life would have been different if we were not able to synthesize carbon-carbon bonds. Imagine a life without daily life materials!
Nobel prize in chemistry
Magnesium metal dissolves in ether in the presence of bromoalkane was found by Victor Grignard in 1900. The “Grignard reagent” that resulted from the reaction reacted with certain types of molecules to form new products. This reaction was later named as Grignard reaction and was immediately a hit. Grignard received Ph.D. from the University of Lyon in 1901 and was granted Nobel Prize in Chemistry
after 11 years, at the age of 41.The Grignard reaction received universal recognition since then. It shapes the world of organic chemistry today. But the molecular mechanism behind the Grignard reaction was unknown until now. The lack of knowledge about the reaction prevented scientists from developing new ways to optimize it.
Professor Michele Cascella decided to have a closer look at the reaction when Professor Odile Eisenstein presented the Grignard reaction as a reaction that is too complex to be understood during a seminar at the University of Oslo. This led to a collaboration.
The three-dimensional structure of the Grignard reagent was difficult to determine even when its composition is known. In fact, it is believed that the structure of the Grignard reagent continuously change into another through a process called “Schlenk equilibrium.” Different groups linked to the central magnesium atom and by the solvent influence this equilibrium.
Cascella and Eisenstein used computer simulations to tackle the problem. They were able to detect the multiple chemical species during the Schlenk equilibrium by modeling both the solvent and reagent in a realistic manner.
They found that the solvent molecules that combine to or detach from, the magnesium atoms determine the whole process. Thus the exchange of partners for the magnesium atom is driven by the dance of solvent leading to Schlenk equilibrium and different compounds.
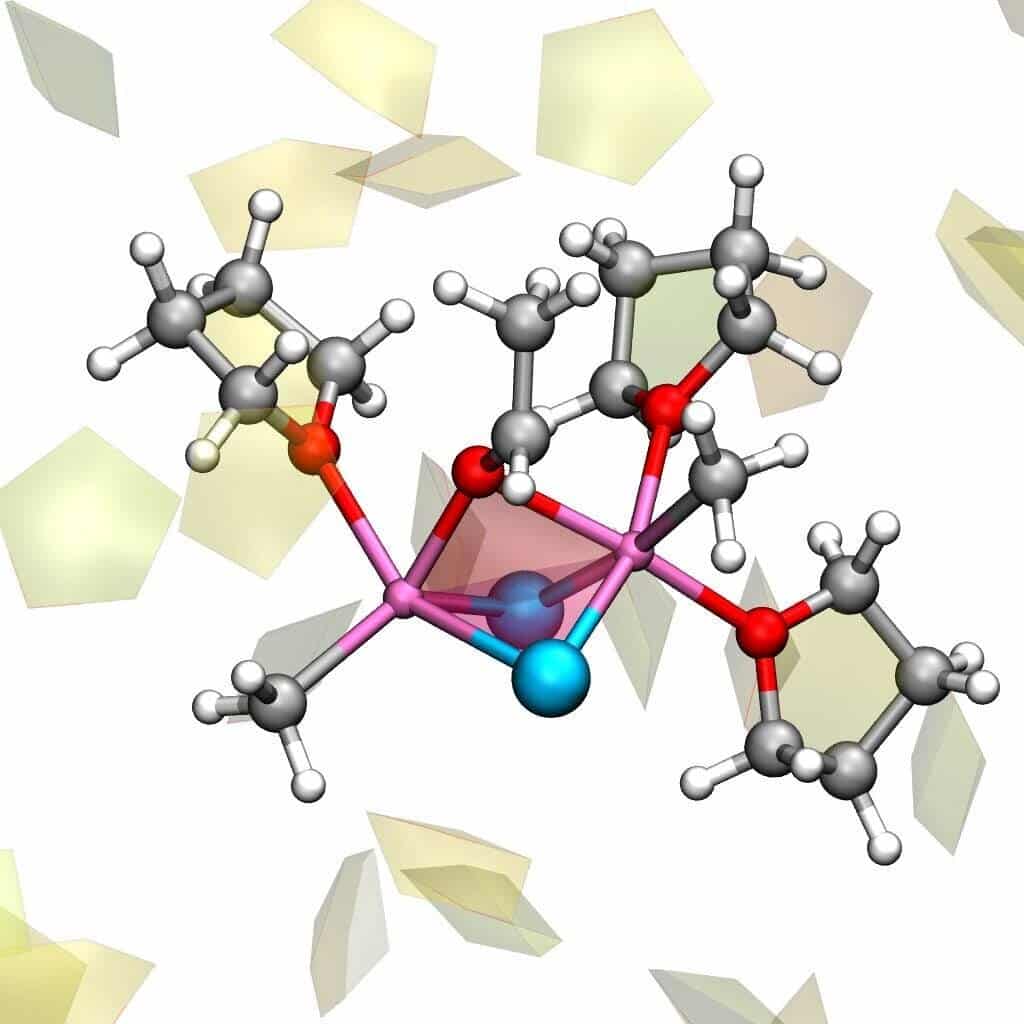
The dance of the Grignard reagent
It became possible to look at the reaction after knowing that Grignard reagent an ever-changing dancer rather than a single well-defined compound. This raised many questions like what compounds present in solution are truly reacting, and how?
High-level quantum chemistry data accompanied by computer simulations enabled Professor Jürgen Gauss (Johannes Gutenberg-University Mainz, Germany) to establish a series of key points.
First, all the molecules produced by the Schlenk equilibrium promote the formation of carbon-carbon bonds, although at different rates.
Second, different substrate molecules will react following different mechanisms characterized by either heterolytic or homolytic splitting of the magnesium-carbon bond (the two electrons of the bond go to the carbon, or are equally shared between the magnesium and the carbon)
Cascella described Grignard reaction as a group of reactions that occur at the same time in the same sample. In the Grignard reaction, the solvent drives the whole chemical process, unlike other common reactions.
The identification of the mechanism of the reaction is just the beginning of the story. Now as the mechanism behind the Grignard reaction has unveiled, scientists can construct from it to understand how the additives like salts, derivatives of other metal compounds, etc enhance organometallic reactions.
The new discovery can help scientists to improve the reaction for the synthesis of molecules needed in industry and medical chemistry. Scientists unexpectedly found that the most reactive species is similar to the active site of a group of enzymes called endonucleases, which are crucial for our existence.
Endonucleases use magnesium as the key factor to catalyze bond formation/breaking in DNA, just like in the Grignard dance. This opens up the possibilities to understand the evolution of enzymes as well as to improve the Grignard reaction itself.

















































