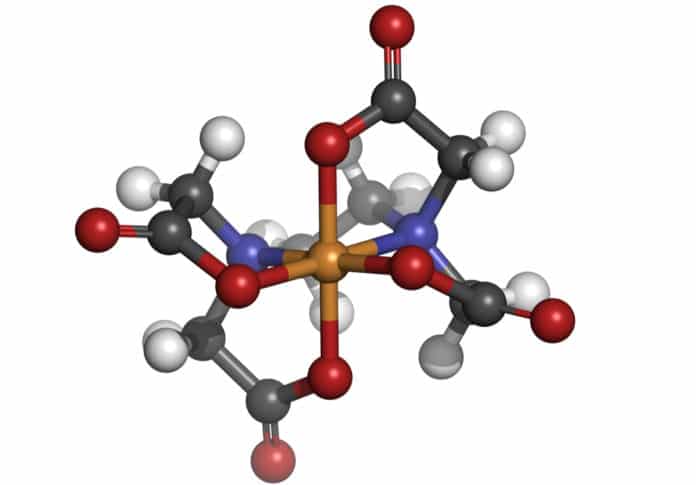
New Molecular Structure Found
Imperial College, London Researchers have reported a transition metal complex with a geometric arrangement of atoms that was predicted in 1893 by the 1913 Nobel Prize recipient.
This important development in inorganic chemistry demonstrates the existence of hexagonal planar geometry in a transition metal complex with potential significance for catalysis, synthesis, materials science, photophysics, and bioinorganic chemistry.
Dr. Alison Edwards said that Six coordinate complexes are ubiquitous in coordination chemistry. Remarkably, Alfred Werner in his seminal research in the 19th and early 20th century, using only observable properties, established the existence of various possible isomers for six coordination and derived three proposed geometries.
New Molecular Structure Found- The Previous Prediction
These late 18th century observations predated X-ray diffraction studies by 20 years and Werner received the Nobel prize for his studies the year after Laue received the Nobel Prize for his observation of the diffraction of X-rays by crystals.
The investigators suggested that their findings had the potential to introduce new design principles for transition metal complexes with implications across the physical and biological sciences.
Data from the Koala Laue diffractometer at ANSTO verified the hexagonal pyramidal coordination environment of a seven coordinated nickel complex closely related
to the hexagonal planar complex which has a palladium atom surrounded by three hydrides and three magnesium atoms.
All of the spectroscopy, X-ray crystallography and theoretical calculations, used to characterize the structures, were done at Imperial College.
The group identified the possible location of the hydrides early in their work from electron density apparent when modeling the structure from X-ray diffraction data.
New Molecular Structure Found- The Future Scope
Scientists highlighted that the experiments were quite challenging as the crystals were air and moisture sensitive therefore they used an inert nitrogen gas atmosphere at low temperature to prevent sample degradation. The neutron diffraction experiment confirmed that the addition of one more ligand to the six-coordinate complex pushes the six coordinating ligands away from the metal plane that the new ligand bonds to, while the hexagonal array is retained.
Collaborating organizations included Imperial College London, University of Oxford and ANSTO.
Author: Rahul Mishra