Researchers Propose a Novel Algorithm for Chirality Determination
Scientists at the Molecular Photonics group of the Van “t Hoff Institute for Molecular Sciences at the University of Amsterdam have now significantly improved the experimental determination of the chirality or say handedness of the molecules using vibrational circular dichroism (VCD) spectroscopy. Scientists, by employing a genetic algorithm they were able to tame the uncertainties in vibrational circular dichroism analysis resulting from the fact that the flexible molecules can adopt many of the structural conformations. This improvement by the research team could see vibrational circular dichroism applied on a large scale, for instance even as a tool for high-throughput screening of pharmaceutical compounds or the real-time monitoring of various biochemical processes.
The research team led by professor Wybren Jan Buma publish their novel algorithm for chirality determination i.e, novel vibrational circular dichroism VCD methods on the 7 September issue of Chemical Science which is the flagship journal of the Royal Society of Chemistry.
According to Mark Koenis, the first author and a Ph.D. student said that it is now possible to determine the handedness or chirality of the molecules much more reliably and even with better quantitative measures than ever before.
In
their research paper, Buma and his team demonstrate their novel approach with a novel algorithm for chirality determination, amongst others, by studies on citronellal. This is a typical example of the class of molecules that have until now posed challenges i.e, often insurmountable to VCD analysis. Chiral, with a meaning that it can exist as two molecular structures which are non-superimposable mirror images of each other which is just like our right hand and a left hand. It is also a very flexible and a dynamic molecule which can adopt many different spatial structures, that are called as conformation.Being chiral, citronellal represents a class of the molecules with great biochemical and pharmaceutical relevance. Since many of the biological molecules like proteins, receptors, enzymes, etc. are chiral, the handedness of the chiral molecules determines their biological interactions. When it comes to the citronellal, its chiral mirror structures that are called enantiomers differ in their interaction with olfactory receptors so that the left-handed molecule smells of oranges and its right-handed counterpart smells of lemons. Even in many other molecules, the effect of chirality can be much more dramatic than this. In many of the pharmaceutical applications, for example, one enantiomer of a drug may have a beneficial therapeutic effect, while the other has harmful biological consequences.
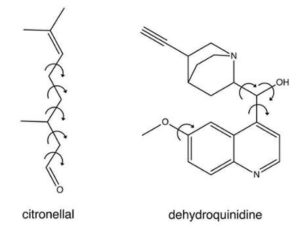
Being very flexible and dynamic, citronellal molecules illustrates the challenges of chirality or handedness determination by means of vibrational circular dichroism spectroscopy. Vibrational Circular Dichroism makes use of circularly polarized light which in fact displays a handedness or chirality in the difference between their left and right circular polarization. Thus, this enables to distinguish between the left- and the right-handed molecules. This sophisticated technique yields a spectroscopic fingerprint which is unique for each molecule and even for each mirror image of the same molecule. For all practical purposes, Vibrational Circular Dichroism is the only technique that is capable of distinguishing between the enantiomers under real-life condition.
Just like citronellal, there are many molecules which are flexible and dynamic, adopting different spatial structures. And each structure has its own fingerprint so that an actual Vibrational Circular Dichroism spectrum is the total of all these fingerprints of all spatial molecular variants present in the sample. In addition to this, more stable and low-energy variants will be more present than the higher-energy ones so that not all variants can contribute equally to the Vibrational Circular Dichroism spectrum. Here the structural freedom forms serious problems for determining chirality in most of these cases.
The customary solution is the novel algorithm for chirality determination i.e, Vibrational Circular Dichroism analysis is to determine all possible conformations of molecules under investigation, calculate their energies and even corresponding fingerprints, and then average these individual components, fingerprints and then compare the resulting spectrum with the experimental Vibrational Circular Dichroism spectrum.
However, this is much less clear-cut than it might appear. And many methods are available for the calculation of energies of various spatial structures, from a very simple to very advanced one. According to Buma, in the worst case scenario, it might be that one type of calculation which would lead to the conclusion that these molecules have one particular type of handedness or chirality, while another type of calculation may lead to the opposite conclusions.
Buma and his team have now significantly improved the ways to calculate and compare, that is a strategy by explicitly taking the uncertainty in the calculated energies into account. Using a genetic algorithm which uses the principles of evolution as well as the survival of the fittest by which they could adjust the contributions of the various fingerprints in such a way that the best agreement with that of the experimental Vibrational Circular Dichroism spectrum was obtained. The beauty of this approach is that the correct handedness always leads to better agreement with the experimental data than the opposite handedness, said, Koenis. Koenis also added that even more importantly, this enables us to present a quantitative measure of the reliability of the novel algorithm for chirality determination using Vibrational Circular Dichroism assignment.
The genetic algorithm which is a novel algorithm for chirality determination was not only tested on citronellal but also on dehydroquinidine molecule, a chiral molecule representing a worst-case scenario because it shows large dynamic structural changes.
Moreover, the Vibrational Circular Dichroism spectrum of dehydroquinidine is experimentally much harder to obtain and the available spectrum is therefore of a much lower quality than what is normally aimed for. The results show that even for such difficult molecules the novel approach is by far superior to all existing methods for absolute configuration assignment.
The researchers expect that the novel algorithm for chirality determination and their improvement of the reliability of VCD as an analytical tool will bring applications within reach such as quality control in the production of pharmaceutical ingredients. They have already performed studies to determine levels of chiral impurities using VCD. Researchers have also shown that notoriously difficult problems such as molecules with many chiral centers can be tackled, said Buma. Taken into consideration that VCD is experimentally more simple and cost-effective than other techniques, he foresees increasing opportunities for application of the technique both in development and large-scale production of chiral molecules.
Author: Ria Roy