Ball-Milling: A Green Technology To Breakdown & Recycle Polystyrene
Researchers from Ames Laboratory, the U.S. Department of Energy have found an eco-friendly, low-energy method to disintegrate polystyrene, a plastic-type that is extensively utilized in cutlery, disposable food boxes, foam packaging materials, and several other uses.
Polystyrene belongs to a much bigger picture of the worldwide plastic litter conundrum. Thousands of million metric tons of plastic types are manufactured annually, a huge portion of those are disposed of after use. Because of the durability and chemical stability of industrial polymers, plastic litter doesn’t simply break down in landfills and is mostly combusted, which generates CO2 and other harmful gases. For stopping the increasing polymer waste and reducing CO2 emissions, plastics need to be converted into new value-added items or have to be recycled.
Presently, recycling this enormous quantity of polymers is not economically possible. Separating and sorting plastics itself is laborious and time-intensive and along with that remanufacturing and chemical processing need a lot of harmful solvents and energy input. Inferior qualities are exhibited by the re-processed polymers mostly than the newly produced made from scratch products.
A group of researchers from Ames Laboratory employed processing by ball-milling to dismantle industrial polystyrene in one step
in the ambient atmosphere at room temperature and devoid of toxic solvents. Ball-milling is an approach that positions materials inside a milling vial along with metal ball bearings which is later agitated till a preferred chemical reaction happens. This is known as mechanochemistry. This experimental technique has various applications in new product manufacturing and interesting quality where plastic recycling is concerned.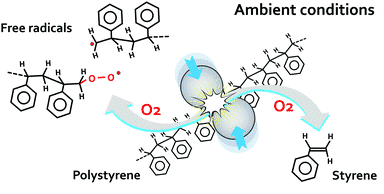
The disintegration of polystyrene proceeds via a series of chemical occurrences involving mechanical fragmentation of bigger molecules, which produces free radicals visible within the milled matter even after the extended subjection to air. The ambient oxygen and metal bearings utilized for milling serve as co-catalysts that allow retrieval of monomeric styrene from oligomeric radical-bearing matter generated. The experiments indicated that the rise in the temperature within the material in the midst of milling is not the reason for the discovered phenomenon as the temperature within the miller powder doesn’t go beyond 50 °C whereas the thermal decomposition of the plastic in the air begins at around 325 °C. Clemson’s team verified that the extensive disintegration of the actual polymer to smaller matter, and oligomeric fragments, suitable for more processing into new value-added items.
Viktor Balema, Senior Scientist, Ames Laboratory stated that this approach represents a significant breakthrough that allows disintegrating a polymer concurrently with its break down at ambient environments, that is, ~300 °C lower than the thermal decomposition condition for the intact material. They suppose this experimental concept is a thrilling opportunity for producing new recycling methodologies for all types of polymers, and that will eventually contribute to the establishment of an eco-friendly economy.
Prof. Igor Luzinov, Kentwool Distinguished Professor, Clemson University, and Balema’s associate reported that this breakthrough opens up new paths for low-temperature retrieval of monomers from multicomponent polymer-based systems like laminates and composites. Additionally, their technology will enable the extraction of the monomer from crosslinked materials comprising styrene units in their framework.
Prof. Aaron Rossini, Alfred P. Sloan Foundation Research Fellow, Iowa State University, added that the Huge concentration of free radical carbon-based species within polystyrene was shown by EPR (Electron Paramagnetic Resonance) spectroscopy that was milled in air. As free radicals are generally extremely reactive, this is an astonishing result. Further, the radicals’ presence radicals provide direct proof that milling directly results in fragmentation of the polymer chains. They anticipate that the reactive spots linked with free radicals can be employed to functionalize the modified polymers to produce new value-added items.
The study is more described in the article titled ‘ Depolymerization of polystyrene under ambient conditions’ written by Igor Luzinov, Aaron J. Rossini, Oleksandr Dolokto, Mastooreh Seyedi, Scott L. Carnahan, Ihor Z. Hlova, and Viktor P. Balema. This study is presented on the cover page of the New Journal of Chemistry.

Green Technology To Breakdown Polystyrene, Recycle polystyrene, Recycle polystyrene
Read Exciting Chemistry & Pharma News At Rasayanika

















































