Pharmacovigilance as a Career: Guarding Drug Safety
Visualize a world where a tragedy can be prevented by careful observation. Some silent heroes notice even the most minor signs of danger hidden in a drug, and behind every safe drug prescription lies this hero. This isn’t Drug Discovery or any treatment, but of unwavering vigilance, a profound sense of responsibility, and the profound impact of protecting lives when risk hides in plain sight. Welcome to a career where your attention and dedication become the invisible shield between harm and healing.
Pharmacovigilance (PV) is more than a Regulatory function—it’s a critical public health endeavor. By definition, PV is the science and set of activities related to detecting, assessing, understanding, and preventing adverse effects or other drug-related problems.
Drugs don’t stop evolving even if it has reached the global market. Once a drug or a therapy is launched for the public, it interacts with real-world scenarios, unforeseen conditions, and a diverse range of populations, beyond what Clinical Trials can simulate. This is where Pharmacovigilance steps in, to monitor the safety profiles of drugs continuously as well as ensure that their risks do not outweigh the benefits of drugs. For example, a drug that was safe in a controlled and monitored Clinical Trial environment could cause ADRs (Adverse Drug Reactions) in ailing patients with certain Genetic profiles or when consuming specific medications and treatments. PV professionals are responsible for managing and identifying such ADRs.
With the rise of Personalized and customized Therapies, accelerated drug approvals, as well as Biologics, PV has become a strategic pillar in the Healthcare systems and Biologics sectors globally.
Why Pharmacovigilance Matters Now More Than Ever
A Drug’s journey doesn’t end with the Regulatory approval or the launch. During the Drug Development process, Clinical Trials occur under tightly controlled conditions and are quite rigorous. Once a novel Drug enters the real world market, it interacts with people with diverse lifestyles, Genetic profiles, as well as associated medications, the scenarios that Clinical Trials can’t fully predict. This is where PV steps in, ensuring ongoing safety surveillance through the analysis and collection of real-world data, enabling the detection of long-term, population-specific, as well as rare ADRs. From preventing tragedies such as the Thalidomide crisis (1950s) to the withdrawal of Vioxx (Rofecoxib) due to cardiovascular risks, these professionals aid and protect millions of lives.
From Compliance to Strategy
Earlier, Pharmacovigilance was considered a mere Regulatory checkbox, but now it has become a strategic Drug Development and Healthcare. With the evolving rise of Gene Therapies, Biologics, and fast-tracked drug approvals, continuous safety assessment is essential.
Modern PV professionals don’t just review case reports; rather, they interpret the Health trends, collaborate with Regulatory and Clinical teams, analyze Big Data, and support benefit-risk decisions that directly influence a drug’s lifecycle.
PV offers a purpose-driven and dynamic career path for those passionate about Life Sciences, public well-being and health, as well as Data.
A Career Built for the Digital Era
The global PV market is expected to reach $24.76 billion by the year 2030, with growing drug consumption, Digital Healthcare transformations, as well as stricter Regulations.
Wearables, AI-powered diagnostics, RWE (Real-World Evidence), and Health applications feed massive volumes of safety data into global databases. India has emerged as a leading Pharmacovigilance hub, with companies like IQVIA, Biocon, Parexel, and Accenture outsourcing significant portions of their PV operations to cities like Bengaluru, Hyderabad, Mumbai, and Gurgaon.
This technological evolution is creating demand for professionals who understand Pharmacology and can work with Data Analytics, ML (Machine Learning), and digital platforms.
The Role of a Pharmacovigilance Professional
Pharmacovigilance professionals ensure that medicines remain as safe as possible throughout their lifecycle. Pharmacovigilance professionals work with multiple responsibilities to ensure drug safety across global populations. A typical day might include:
- Monitoring and Collecting ADRs: From Healthcare professionals, patients, Literature, or spontaneous reporting Databases.
- Signal Detection and Benefit–Risk Analysis: Identifying emerging trends and potential safety signals from large Biological Datasets.
- Processing ICSRs (Individual Case Safety Reports): Using industry-standard tools like Oracle Argus, ArisG, or Veeva Vault Safety.
- Regulatory Authorities: Interacting with authorities, including the FDA (Food and Drug Administration), EMA (European Medicines Agency), CDSCO (Central Drugs Standard Control Organisation), and MHRA (Medicines and Healthcare Products Regulatory Agency), to ensure compliance, timely safety reporting, and evolving drug safety requirements.
- Authoring Regulatory Safety Reports: These include PSURs (Periodic Safety Update Reports), DSURs (Development Safety Update Reports), as well as RMPs (Risk Management Plans).
Beyond their technical duties, PV professionals act as Ethical stewards, as they balance commercial Drug interests with public health imperatives and uphold a safety-first culture within the Pharmaceutical and Biotechnology industry.
Key Skills to Succeed in Pharmacovigilance
A foundational degree in Medicine, Pharmacy, Life Sciences, or Biotechnology is a good starting point, but thriving in Pharmacovigilance demands a blend of Analytical, Technical, and Soft skills:
- In-depth knowledge of Medical Terminology as well as Pharmacology
- Strong critical thinking and pattern recognition for detecting safety signals
- Precision in Documentation as well as Scientific Writing
- Familiarity with global Regulatory Guidelines such as the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use), WHO-UMC (World Health Organization – Uppsala Monitoring Centre), and MedDRA (Medical Dictionary for Regulatory Activities).
- Proficiency in PV Software Tools and Safety Databases
- Effective communication for liaising with cross-functional and global teams
- Awareness of emerging tools like AI (Artificial Intelligence), RWE (Real-World Evidence), and Machine Learning in signal detection
Today, professionals exposed to Data Analytics, AI, as well as Digital Health technologies are at the forefront of advanced PV roles. Professionals who continuously upskill are leading the next generation of Pharmacovigilance, ensuring that the field remains dynamic and exciting.
Career Paths in Pharmacovigilance
Pharmacovigilance offers a structured and rewarding career progression, especially for those willing to upskill and adapt. Typical entry-level and advanced PV roles include:
|
Entry-Level Roles |
Mid-Senior Level Roles | Advanced Roles |
|
Drug Safety Associate |
Signal Detection Analyst |
Pharmacovigilance Director / Manager |
|
ICSR Processor |
Aggregate Reports Specialist |
Director of Safety / PV Scientist |
| Case Quality Reviewer | Medical Reviewer |
Global Safety Leader |
As your experience grows, you may transition into Regulatory Affairs, Clinical Development, Quality Assurance, or AI-based Pharmacovigilance strategy roles.
Where Can You Work?
The scope of Pharmacovigilance is global, and your career can take you across continents, offering diverse and exciting international career opportunities. Employers include:
- Pharmaceutical & Biotechnology companies: Global Pharmaceutical companies such as Roche, Pfizer, AstraZeneca, as well as Novartis, drive Drug innovation while depending on PV teams to ensure Regulatory Compliance as well as ongoing Drug safety worldwide.
- CROs (Clinical Research Organizations): Companies such as IQVIA, Covance, and Parexel manage outsourced PV projects, providing diverse exposure to Drug safety operations globally.
- CSOs (Contract Safety Organizations): These organisations specialize in providing expert PV services, focusing on efficient safety reporting as well as case processing across multiple clients.
- Health tech startups & AI companies in Drug safety: Innovative startups utilize digital and AI tools to transform PV through Predictive Analytics as well as automated Drug Safety Monitoring.
- Government and Regulatory bodies: Regulatory agencies like CDSCO, FDA, and EMA safeguard public health by enforcing drug safety standards and overseeing compliance.
- Academic and public health institutions: These institutions conduct Research and surveillance to identify drug safety trends and inform public health policies globally
Leading companies such as CliniOps, Covance (Labcorp), Biocon, as well as APCER Life Sciences, offer exciting opportunities in India. India has become a preferred outsourcing destination for global Pharmacovigilance activities, with hubs in cities including Hyderabad, Bangalore, Gurgaon, and Mumbai.
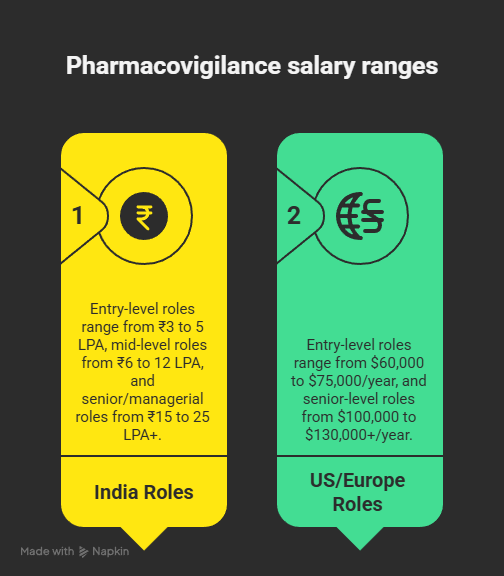
Salary Insights & Growth Potential
Pharmacovigilance is a fast-growing, high-demand field that offers both stability and career mobility:
- India:
- Entry-level roles: ₹3 to 5 LPA
- Mid-level roles: ₹6 to 12 LPA
- Senior/Managerial roles: ₹15 to 25 LPA+
- US/Europe:
- Entry-level roles: $60,000 to $75,000/year
- Senior level roles: $100,000 to $130,000+/year
The global nature of PV means experienced professionals often enjoy opportunities to work on international projects or relocate for better roles.
Getting Started: Education & Certification
Most employers look for a Bachelor’s or Master’s degree in Pharmacy, Biotechnology, Medicine, Life Sciences, or Microbiology. However, to break into this competitive field, some additional certifications help:
- Postgraduate Diploma in Clinical Research & Pharmacovigilance.
- Certified Professional in Pharmacovigilance (CPPV).
- MedDRA and ICH-GCP training.
- Hands-on training in PV tools such as ArisG, Veeva Vault, and Oracle Argus.
Institutions such as Biotecnika, DIA (Drug Information Association), Symogen, ICRI (Institute of Clinical Research India), as well as Regulatory training centers, offer PV industry-relevant courses, like Regulatory Compliance, Database training, and real-world case study analyses.
Pharmacovigilance Future Trends
Pharmacovigilance is being reshaped by digital health and global innovation. Here are key trends to watch:
- Patient Engagement: Real-world experience feedback loops and Direct-to-patient safety reporting.
- Wearables and IoT: Real-time adverse event monitoring through connected health devices.
- AI-Driven Case Processing: Signal detection through Machine Learning and Automated case triage.
- Global Harmonization: Efforts by WHO, ICH, and national agencies to standardize PV practices
- Blockchain: Enhanced data security and traceability in PV systems
As these innovations unfold, the PV professional of tomorrow will need to be part regulator, part advocate for patients, as well as part data scientist.
Conclusion
Pharmacovigilance is more than a career; it’s a commitment to humanity. Every report processed, every regulatory update, and every signal detected and issued contributes to a world where Medicine heals without harm.
Pharmacovigilance offers the perfect avenue if you’re a Life Sciences graduate seeking an intellectually fulfilling, purpose-driven, as well as globally relevant profession. It bridges Science with service, Technology with trust, and data with decisions.
Ultimately, while Doctors prescribe and Chemists or Pharmacists dispense, the Pharmacovigilance professional ensures the Drug’s story ends well!