Table of Contents
Material Chemistry and Its Role in Developing Advanced Molecular Filters
Imagine a tiny molecular sieve that is so precise that it can capture molecules from air or water. This is not a science fiction movie, but modern material chemistry is tailoring advanced materials that can act as molecular filters, materials that can conduct electricity, and resist corrosion, etc.
Material Chemistry is the study of the structure and properties of materials at the atomic or molecular level, such as permeability, conductivity, and strength, so that they can be modified or designed to create materials that work the way we want them to.
One groundbreaking innovation of applied chemistry is the discovery of the molecular filter. These filters are made up of specialized materials that have the ability to separate molecules or atoms selectively at the microscopic level. This field is shaping the way researchers and scientists design materials with precision and purposeful properties.
In this article, we will discuss how modern chemistry and applied chemistry are changing the world by creating new materials using photochemical strategies to develop advanced materials like a molecular filter, which can be used in bioremediation techniques to purify water and air by separating harmful chemicals like PFAS (forever chemicals)
What are Molecular Filters?
Molecular filters are advanced porous materials that are like a sieve, but different from a conventional sieve because they are engineered to separate substances at molecules or atomic level, allowing a specific molecule or atom to pass through and blocking the other. This is based on charge, size, and shape or chemical affinity. They can be classified into the following types:
- Porous materials
Materials like zeolites and metal-organic frameworks (MOFS) are called porous materials and have defined pores that trap specific molecules by absorbing them. Such materials allow the separation of gases, liquids, and ions.
- Polymeric membranes
Polymeric membranes are manipulated to engineer materials to induce durability, permeability, and selectivity.
- Photochemical filters
These filters are incorporated with components or catalysts that respond to light, and they activate chemical reactions when they are exposed to light. These chemical reactions help in degrading pollutants and improve the efficiency of filtration.
Photochemical process to make Molecular Filters
Material chemistry directly influences the filtration properties of a material because it helps in the design and incorporation of molecules or atoms to achieve desired properties, such as pore size and permeability at the molecular or nanoscale level.
Photochemical methods are used to incorporate functional groups on the surface of filters using chemical reactions, using light-driven methods because the chemicals get activated in the presence of light. The photochemical reaction approaches are:
- Chemical reactions that are activated by light
Materials are induced with functional groups like OH, NH2, or -COOH, which get activated or form hook-like structures when the material is exposed to light. This change in the material enhances its trapping ability by activating the functional group to bind with the pollutants and capture them.
- Photolytic filters that degrade pollutants
These filters have a photocatalyst that reacts when exposed to light and breaks down organic pollutants into harmless substances like Carbon dioxide and water. Light provides energy to the photocatalyst to break chemical bonds in pollutants, which they absorb and decompose.
Some Advanced Materials in Modern Chemistry for Filtration
The advanced technologies applied in chemistry have enabled scientists to develop smart materials that have enhanced filtration properties. These also include supramolecular systems, nanomaterials, and other hybrid materials.
- Supramolecular systems: They design materials focusing on non-covalent interactions of molecules or atoms that can recognise specific molecules and trap them. They focus on non-covalent interactions like hydrogen bonding andelectrostatic forces.
- Nanomaterials: They have high surface area, mechanical strength, and improve permeability, which enables fast filtration. e.g., graphene oxide, quantum dots, and carbon nanotubes.
- Hybrid materials: These are a combination of organic and inorganic molecules, which increases the flexibility, strength, and chemical stability in one material. This makes them durable and high-performance filters.
Applied Chemistry is transforming laboratory-discovered materials into industrial filtration systems. They have unlocked the way a filter works by optimizing, scaling, and testing their performance and developing an industrial application. The materials are highly selective, durable, and efficient. Be ahead of such a revolution by joining our course on the Industrial QA/QC training program.
Case Studies
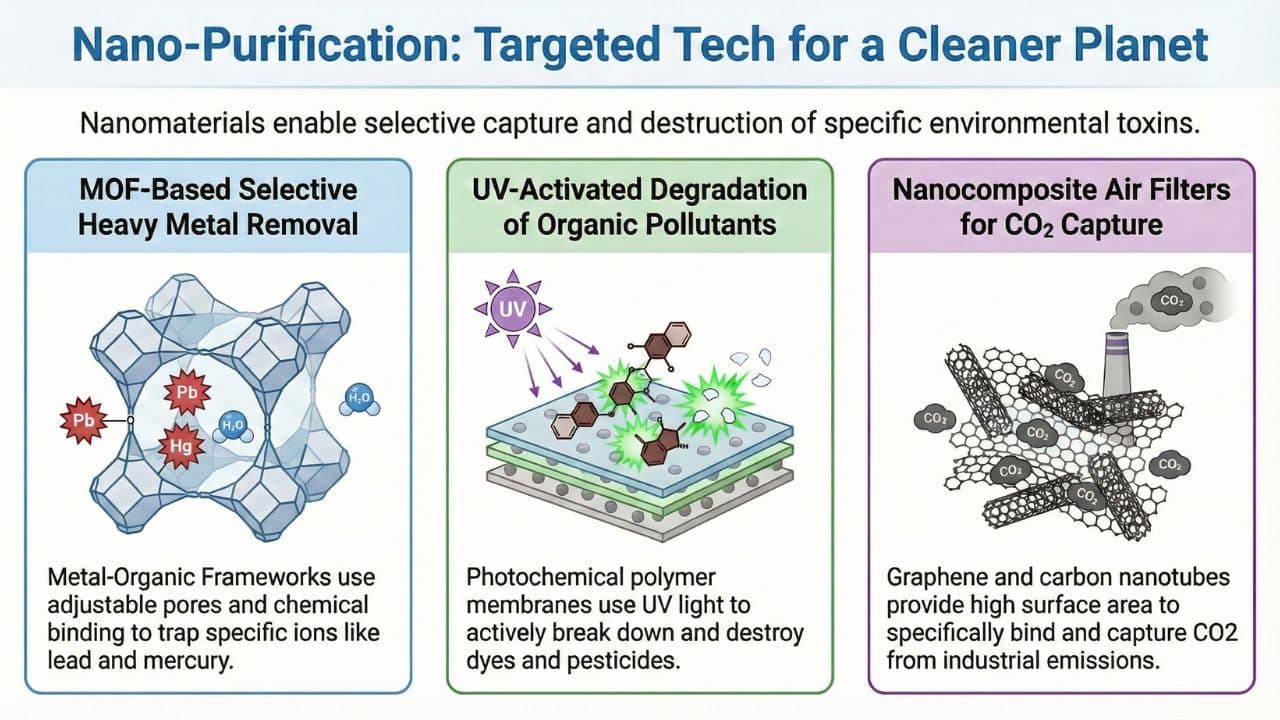
Example 1: MOF-based water filters for heavy metal removal.
These filters are made up of materials that selectively remove heavy metal ions such as lead, mercury, and cadmium, etc from water.
- They have adjustable pores and can fit specific metal ions.
- They have chemical groups on the surface that can bind specific metals through coordination chemistry.
- They have a high surface area, which increases the number of binding sites and allows the efficiency of adsorption.
These filters are highly efficient, as traditional filters cannot remove particles and trap specific metals at the same time. MOF filters remove pollutants by trapping specific molecules and are used to improve the efficiency of water purification.
Example 2: Photochemical polymer membranes for UV-activated pollutant degradation.
These materials are made of polymers, and they degrade organic pollutants such as dyes and pesticides when they are exposed to UV light.
- They have induced a functional group that reacts when exposed to light.
- In the presence of UV light, electrons of the material or embedded photocatalyst get excited or activated and break down pollutants.
This filter not onlycapture them but also destroys them, and they are used in the treatment of water treatment plants and in the treatment of industrial effluent.
Example 3: Nanocomposite air filters for selective CO2 capture.
They are enhanced nano materials, such as graphene oxide, carbon nanotubes, or nanoparticles of metal, to increase the surface area, which increases the adsorption capabilities.
- The functional groups on the surface of the material bind CO2 molecules specifically
- These nanoparticles also increase the strength, durability, and flexibility of the material.
Only pollutants are captured by conventional air filters, and the filters that have nanoparticles can capture CO2. They are usedin industries to control emissions, purify the air indoors, and mitigate climate change.
The advances in applied chemistry and technologies in material chemistry have made it possible to innovate and pioneer lab experiments into real-world applications in the industries. Material chemistry is one of the significant pillars in the development of advanced materials, like molecular filters, because it enables a precise understanding of the material properties, like it structures, compositions, etc. This understanding of the material properties allows a scientist to engineer a material whose structure and chemical behaviour can be manipulated the way they want it to.
Recent improvements in the design of materials, such as the use of nanoparticles and modified materials, have greatly increased the selectivity, efficiency, and durability of filtration systems. These advances have made a strong impact in key areas such as environmental protection, healthcare, and energy technologies.
Modern chemistry helps turn basic molecular knowledge into practical applications, enabling the design of effective filtration systems for industrial use. Ongoing research in material chemistry is essential for further improving selective filtration technologies and for addressing future global challenges.