Table of Contents
Career Shift for Chemistry Postgrads: Why Regulatory Affairs IN 2025 Is Booming
Imagine spending years mastering chemistry, performing countless experiments, and analyzing data, only to feel stuck in a lab doing the same tests day after day. Does this sound familiar? If you’re a chemistry postgraduate looking for a career that combines your scientific expertise with strategy, decision-making, and global impact, Regulatory Affairs might just be your next big step. Regulatory Affairs in 2025 is booming like never before, offering exciting opportunities for chemists to step out of the lab and shape the future of pharmaceuticals, biotech, and beyond. Are you ready to discover why so many chemistry graduates are making the switch? Let’s explore it further.
Why Switch to Regulatory Affairs?
By 2035, the global Regulatory Affairs market will reach over 30 billion dollars, with a significant increase in growth expected to begin in 2025. This rapid expansion will open doors for skilled professionals, especially those with a strong scientific background. For chemistry postgraduates considering a shift from traditional lab work, this booming sector offers a dynamic and intellectually stimulating career path. While chemistry is the backbone of innovation in pharmaceuticals and biotechnology, the world of regulatory affairs is where that innovation is successfully guided to market. This article explores why Regulatory Affairs in 2025 for Chemistry professionals is not just an alternative but a strategic and highly rewarding choice in 2025.
Regulatory Affairs
Regulatory affairs is the department within a company that acts as the link between the organization and government regulatory bodies like the Central Drugs Standard Control Organization (CDSCO) or the US Food and Drug Administration (FDA). The main goal is to ensure that all the products, from the new drugs and medical devices to food additives and cosmetics, are developed, manufactured, and marketed in full compliance with various complex laws and guidelines.
Think of it this way: a team of brilliant chemists might discover a new molecule for a cancer treatment. But, without the regulatory affairs, that molecule can’t be approved for use by patients. The research associates are responsible for preparing and submitting the detailed application packages that prove the product is safe and effective. They manage all communication with the authorities, every step, right from the clinical trials to labeling and marketing, and make sure that the treatment meets strict legal standards. This role is a perfect fit for a Regulatory Affairs Career for chemistry graduates because it requires a deep understanding of the science behind the product.
Why is Regulatory Affairs Booming in 2025?
Several factors are driving the surge in regulatory affairs jobs this year:
- Stricter Global Regulations: Countries are introducing stricter rules on product safety, environmental impact, and clinical testing.
- Rapid Product Innovation: The pharmaceutical and chemical industries are launching new products faster, and there is an increase in the need for timely approvals
- Expanding Global Trade: Companies are entering new markets, requiring regulatory approvals in various regions
- Digital Transformation: Regulatory submissions are moving to electronic platforms, speeding up the process but also demanding skilled professionals.
Because of these trends, jobs in Regulatory Affairs in 2025 for chemistry graduates offer stability that few other fields can match today. Many companies are even hiring fresh postgraduates and training them in-house, knowing that their chemistry background gives them a strong starting point.
Job Market Outlook 2025
The sudden rise in regulatory affairs jobs this year is driven by several factors, such as
- Stricter Global Regulations are being introduced as countries implement tougher rules for product safety, environmental impact, and clinical testing, therefore reflecting a growing emphasis on consumer protection and sustainable practices.
- Rapid Product Innovation in the pharmaceutical and chemical industries is leading to faster product launches, increasing the demand for timely regulatory approvals.
- Expanding Global Trade is pushing companies into new markets, and with growing demand, the need for regulatory approvals is rising across multiple regions.
- Digital Transformation is shifting regulatory submissions to electronic platforms, which speeds up the process while also increasing the demand for skilled professionals.
Because of these trends, a Regulatory Affairs Career For chemistry graduates offers unmatched opportunities for stability and growth in today’s market. Many companies are hiring fresh postgraduates and providing in-house training, recognizing that their chemistry background gives them a valuable advantage.
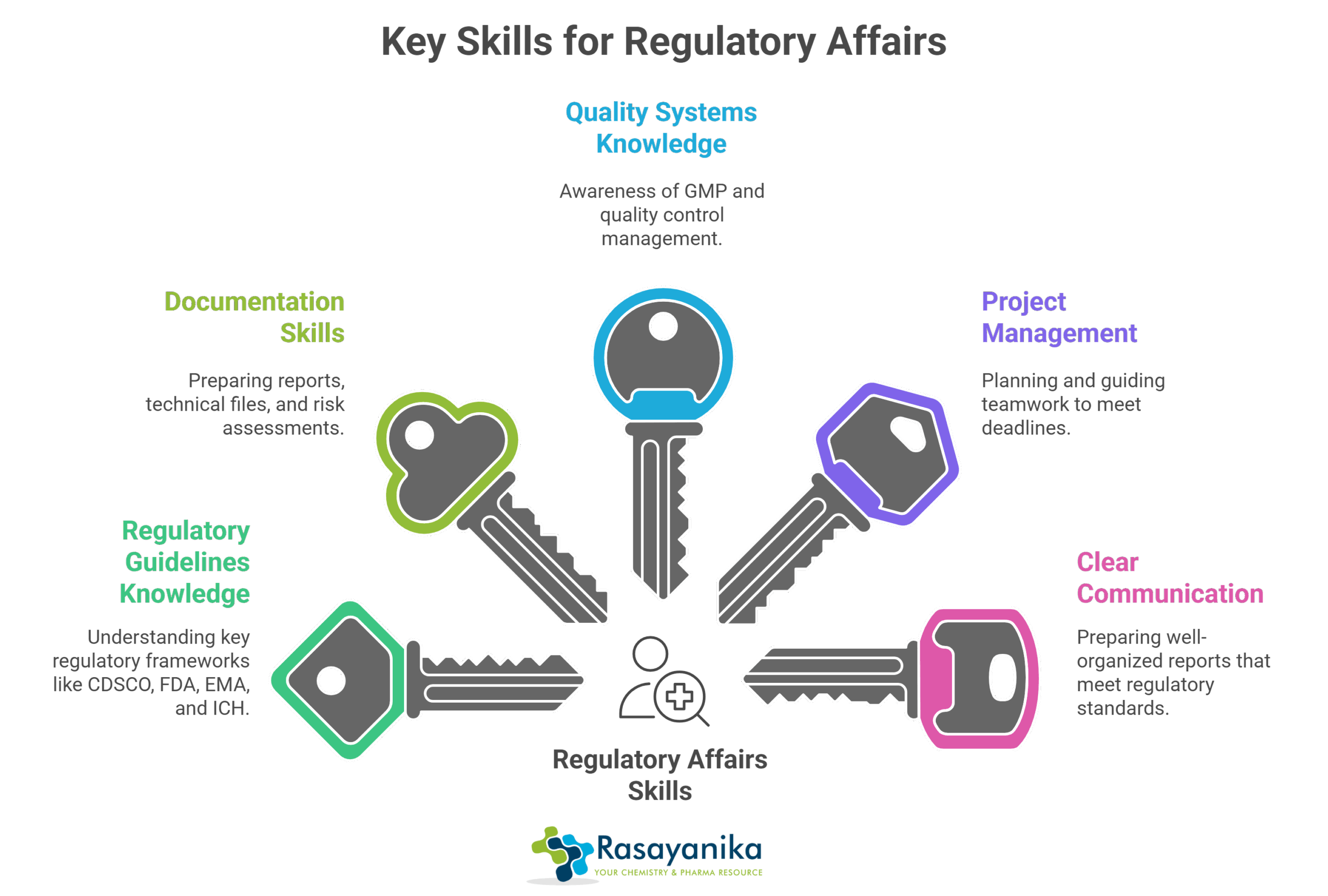
Skills You Need for Regulatory Affairs
Shifting from a purely chemistry-focused role to regulatory affairs involves learning a few key skills and knowledge areas
- Regulatory Guidelines Knowledge involves understanding key frameworks such as the Central Drugs Standard Control Organization (CDSCO), the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonization (ICH) guidelines, etc.
- Documentation skills include preparing reports, technical files, and risk assessments.
- Knowledge of quality systems includes awareness of Good Manufacturing Practice (GMP) and how quality control is managed.
- Project management in regulatory affairs involves planning and guiding teamwork to ensure deadlines are met.
- Clear communication is required to prepare well-organized reports that meet regulatory standards.
Many of these skills can be developed through brief certification programs, hands-on training, or postgraduate courses in regulatory affairs. These developments make it more accessible for chemistry graduates to start a career in Regulatory Affairs in 2025.
How to Grow Your Career in Regulatory Affairs
- A major reason this field is thriving is because of the excellent career growth it offers. Entry-level positions in Regulatory Affairs in 2025 in India offer 5–7 LPA, and with experience, salaries can rise to 15–25 LPA or beyond.
- In the US and Europe, initial salaries are comparatively higher.
- In regulatory affairs, professionals can steadily advance their careers, starting as associates and moving up through specialist, manager, and director roles to become head of regulatory affairs.
- Experienced professionals may also step into higher-level roles, including policy advising and leading global compliance efforts.
- This makes a Regulatory Affairs Career for chemistry graduates not only a stable option but also a highly rewarding one, offering both professional growth and strong financial returns.
How to get started?
If you are a Chemistry postgraduate looking to transition, here are some practical steps to start your career in Regulatory Affairs in 2025
- Take a certification course in regulatory affairs offered by reputed institutes.
- Build your resume around transferable skills such as Scientific Writing, data analysis, and attention to detail.
- Apply for entry-level roles like Regulatory Affairs Associate, Documentation Specialist, or Quality Analyst that are perfect to launch your career.
- Network with industry professionals, attend webinars, workshops, and industry events.
- Stay updated on regulations, follow updates from agencies like the FDA, EMA, and CDSCO.
These steps will make your profile stand out for a Regulatory Affairs Career for Chemistry postgraduates and increase your chances of getting hired.
Challenges You May Face
Like any field, Regulatory Affairs has its challenges
- Since the Rules and regulations keep on changing, continuous learning becomes a necessity.
- Even small mistakes may slow down approvals and result in heavy losses, which is why regulatory affairs is considered a job with serious responsibility.
- Projects usually run on tight schedules for market launches, which makes strong time management an essential skill.
While these challenges may seem demanding, chemistry graduates are well-prepared to tackle them and, in return, get the chance to help bring safe, innovative products to market, a truly rewarding experience.
The Future Path in Regulatory Affairs
In the coming years, regulatory affairs will play an important role. As fields such as gene therapy, personalized medicine, and green chemistry advance, the demand for professionals skilled in navigating complex regulations will increase.
This makes Regulatory Affairs in 2025 an ideal time to consider a career for Chemistry postgraduates. This field combines scientific knowledge with strategic thinking, offering long-term growth and global opportunities.
Shifting to regulatory affairs is becoming a smart option for chemistry graduates, with rising demand, competitive pay, and an international career scope. Companies are actively searching for candidates with scientific expertise, and chemistry graduates match this requirement perfectly.
If you have been wondering where to take your chemistry degree next, consider building a Career in Regulatory Affairs in 2025. It could be the most rewarding move you make in 2025.