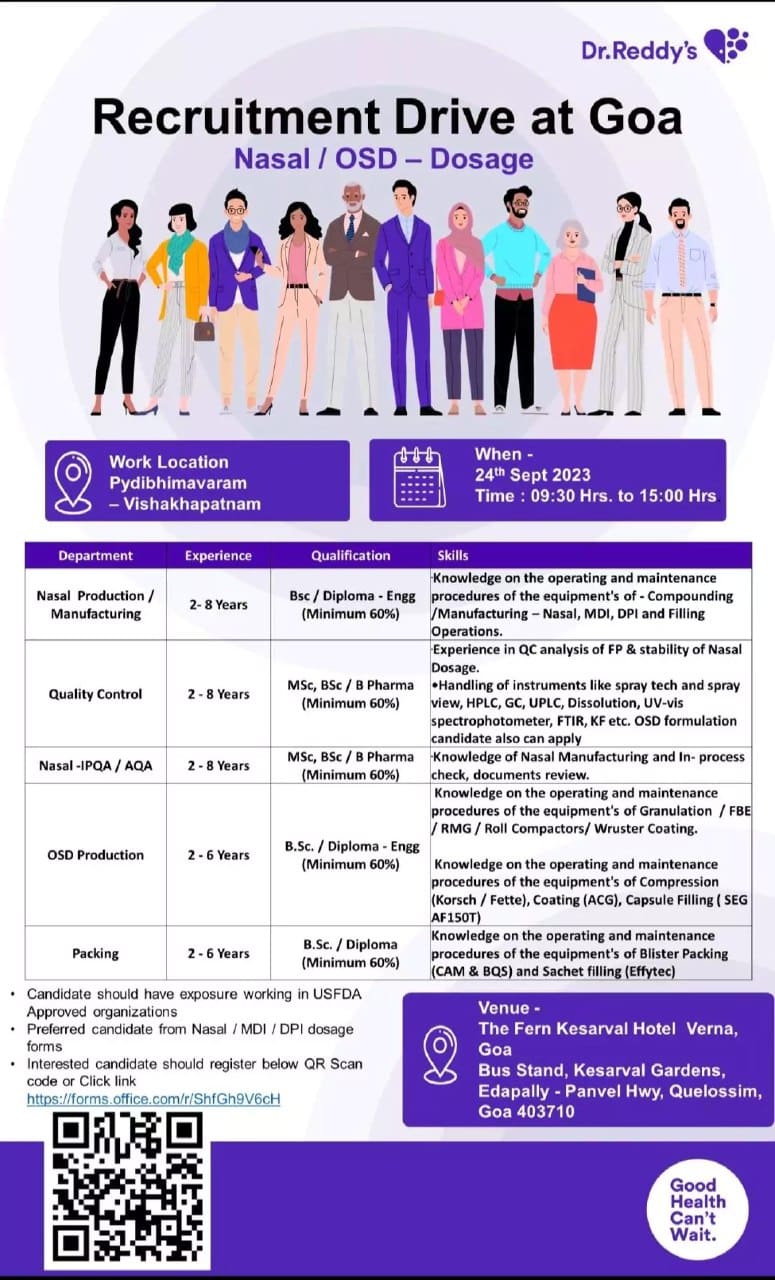
Recruitment Drive at Goa Nasal / OSD – Dosage. We are attaching the details of Dr Reddys Recruitment Drive below. Please go through the same and apply based on your qualifications. Multiple openings at Dr. Reddy’s Laboratories.
Work Location – Pydibhimavaram, Vishakhapatnam
Date and Time – 24th Sept 2023, 09:30 Hrs. to 15:00 Hrs
Venue – The Fern Kesarval Hotel Verna, Goa Bus Stand, Kesarval Gardens, Edapally – Panvel Hwy, Quelossim, Goa 403710
Post 1
Department – Nasal Production / Manufacturing
Experience – 2-8 Years
Qualification – Bsc / Diploma – Engg (Minimum 60%)
Skills – Knowledge on the operating and maintenance procedures of the equipment’s of – Compounding /Manufacturing – Nasal, MDI, DPI and Filling Operations.
Post 2
Department – Quality Control
Experience – 2-8 Years
Qualification – MSc, BSc/B Pharma (Minimum 60%)
Skills –
- Experience in QC analysis of FP & stability of Nasal Dosage.
- Handling of instruments like spray tech and spray view, HPLC, GC, UPLC, Dissolution, UV-vis spectrophotometer, FTIR, KF etc. OSD formulation candidates also can apply
Post 3
Department – Nasal-IPQA/AQA
Experience – 2-8 Years
Qualification – MSc, BSc/B Pharma (Minimum 60%)
Skills – Knowledge of Nasal Manufacturing and In- process check, documents review.
Post 4
Department –
OSD ProductionExperience – 2-6 Years
Qualification – Bsc / Diploma – Engg (Minimum 60%)
Skills –
- Knowledge of the operating and maintenance procedures of the equipment of Granulation /FBE RMG/Roll Compactors/ Wruster Coating.
- Knowledge of the operating and maintenance procedures of the equipment of Compression (Korsch / Fette), Coating (ACG), Capsule Filling (SEG AF150T)
Post 5
Department – Packing
Experience – 2-6 Years
Qualification – Bsc / Diploma (Minimum 60%)
Skills – Knowledge on the operating and maintenance procedures of the equipment’s of Blister Packing (CAM & BQS) and Sachet filling (Effytec)
Note –
- Candidate should have exposure working in USFDA Approved organizations
- Preferred candidate from Nasal /MDI / DPI dosage forms
- Interested candidate should register below QR Scan code or Click the link
https://forms.office.com/r/ShfGh9V6cH
Hello, everyone! We’ve put together a set of interview questions and their respective answers to assist you in your interview preparation for the Dr Reddys Recruitment Drive. Please make use of these resources to ensure a successful performance during your interview for the Dr Reddys Recruitment Drive. Wishing you the best of luck!
For Post 1 – Nasal Production / Manufacturing:
Question: Can you describe your experience in operating and maintaining equipment used in the manufacturing of nasal products?
Answer: During my previous role, I gained hands-on experience in operating and maintaining compounding and manufacturing equipment for nasal products, including MDI and DPI. I ensured that equipment was calibrated, followed standard operating procedures, and conducted routine maintenance checks to minimize downtime and maintain product quality.
For Post 2 – Quality Control:
Question: How have you utilized your analytical skills and knowledge in QC analysis of FP (Finished Products) and stability testing for nasal dosage forms in your previous roles?
Answer: In my previous position, I conducted rigorous QC analysis on nasal dosage forms, including stability testing. I utilized instruments such as HPLC, GC, UPLC, and UV-vis spectrophotometer to ensure product quality and compliance with regulatory standards. This experience has allowed me to develop a keen eye for detail and precision in QC analysis.
For Post 3 – Nasal-IPQA/AQA:
Question: Could you explain your role in In-Process Quality Assurance (IPQA) and your approach to documents review in the context of Nasal Manufacturing?
Answer: In my previous role in Nasal-IPQA, I was responsible for conducting in-process checks to ensure that manufacturing processes met quality standards. I also reviewed documents to verify compliance with procedures. My approach involved thorough inspections, attention to detail, and collaboration with production teams to address any deviations promptly, ensuring product quality and compliance.
For Post 4 – OSD Production:
Question: Can you share your experience in operating and maintaining equipment used in OSD (Oral Solid Dosage) production, including granulation and compression processes?
Answer: In my previous role, I gained valuable experience operating and maintaining equipment for granulation, roll compactors, compression (Korsch / Fette), and other OSD processes. I focused on equipment calibration, routine maintenance, and troubleshooting to ensure the efficiency and consistency of production processes. This approach contributed to improved product quality and reduced downtime.
For Post 5 – Packing:
Question: How familiar are you with the operating and maintenance procedures for equipment used in blister packing (CAM & BQS) and sachet filling (Effytec) for pharmaceutical products?
Answer: I have extensive experience in operating and maintaining equipment for blister packing and sachet filling. I am well-versed in the specific procedures for CAM, BQS, and Effytec machines, ensuring that they operate smoothly, with minimal downtime. This expertise has enabled me to contribute to efficient packing processes and product consistency.