Bristol Myers Careers: Chemical Engineering Jobs
| Apply Now
Are you looking for Product Quality Specialist roles, Chemical Engineering jobs, or exciting opportunities with Bristol Myers Careers? This Specialist position in Global Product Quality Complaint Triage at Bristol Myers Squibb, Hyderabad, offers a meaningful career in pharmaceutical quality management. The role focuses on complaint triage, regulatory compliance, and patient safety while working closely with global stakeholders to support high-quality, compliant products across the BMS network.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company driven by a single mission—transforming patients’ lives through science. Bristol Myers Careers offers impactful opportunities across quality, manufacturing, and Chemical Engineering jobs, including Product Quality Specialist roles. With a strong focus on innovation, compliance, and patient safety, BMS empowers professionals to work on life-changing therapies while growing their careers in a collaborative, values-driven environment.
- Position: Specialist, Global Product Quality Complaint Triage and Network
- Location: Hyderabad – TS – IN
Job description
As the Specialist for Global Product Quality Complaint Triage, you will be responsible for the accurate and timely review, evaluation, and assignment of incoming Product Quality Complaints (PQCs) in a dynamic, high-volume environment. Reporting to the Associate Director of Global Product Quality
Complaints, you will work closely with various stakeholders across the organization to ensure compliant and efficient management of PQCs.Responsibilities
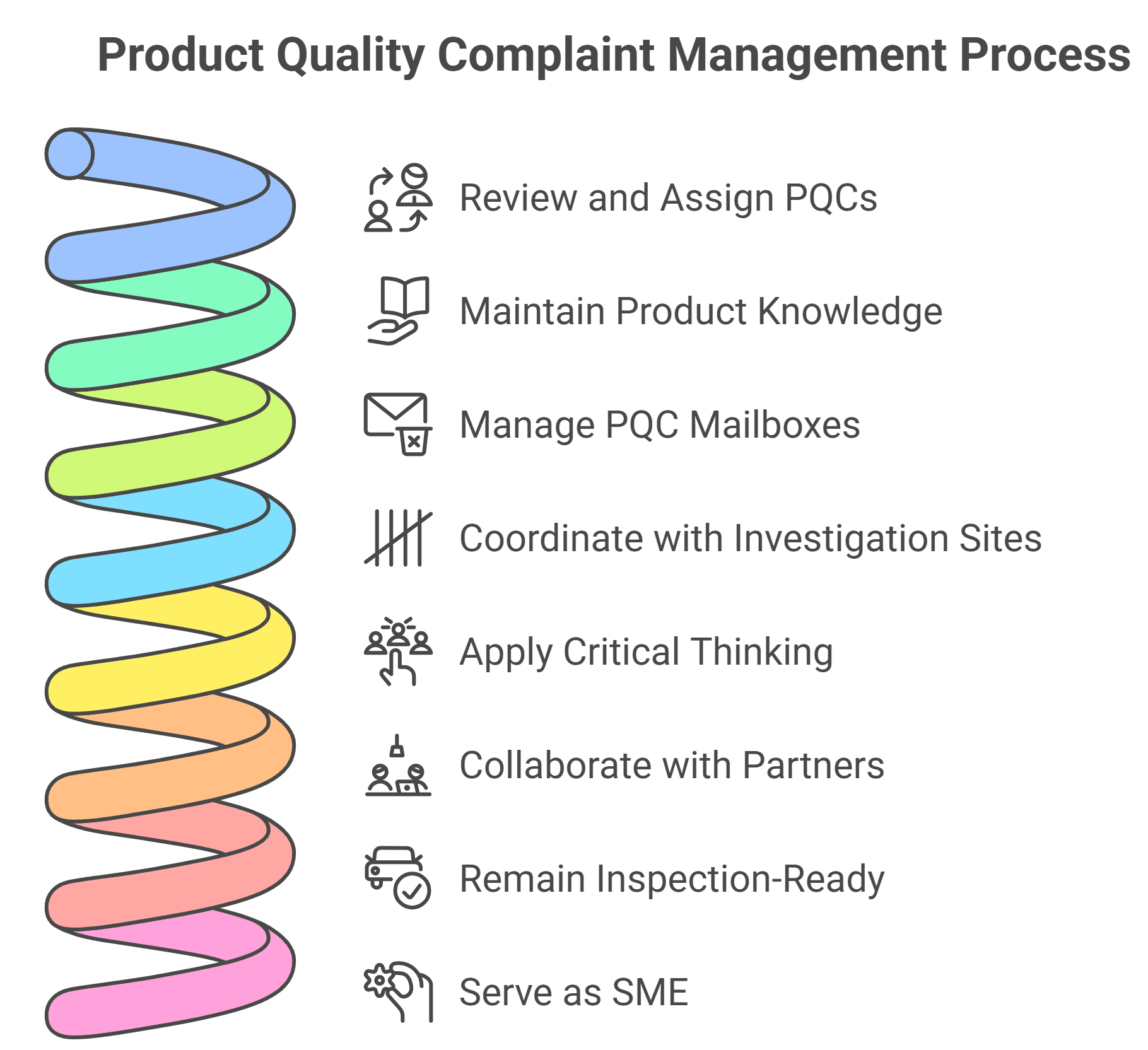
- Responsible for accurate & timely review, evaluation, and assignment of incoming Product Quality Complaints (PQCs).
- Maintain high-level knowledge and understanding of applicable procedures for BMS products.
- Manage PQC mailboxes as a source of intake for PQCs.
- Liaise with investigation sites to deliver high-quality, on-time investigation reports for BMS Quality approval.
- Apply critical thinking in the receipt & review of sample photographs, and in the determination of sample return requirements.
- Engage proactively with upstream and downstream partners to resolve issues and support the overall quality management system.
- Be PQC inspection & audit-ready at all times.
- Act as PQC SME for continuous improvement projects.
Qualifications & Experience
- Bachelor of Science (Life Sciences, Pharmacy, Nursing, Chemical or Bio-Engineering, or related field).
- Fluent in English, with proven professional working proficiency in English.
- In-depth understanding of the global regulatory environment with respect to Product Quality Complaint processing.
- Ability to apply critical thinking in a high-volume, fast-paced environment, with the knowledge that decisions support patient safety.
- A minimum of 3 years of experience in a global Pharmaceutical/Bio-pharmaceutical/Device organization in a Quality role.