Executive Job at Amneal | MSc Chemistry Graduates Apply Now
Are you looking for an Executive Job at Amneal in the pharmaceutical industry? Join our Ahmedabad site as an Executive, IPQA, ensuring cGMP compliance and high-quality production standards. This role is ideal for candidates with MSc Chemistry or related degrees seeking QA/QC Jobs and pharmaceutical career opportunities.
About Amneal
Amneal Pharmaceuticals offers career opportunities through Amneal Careers, providing positions in QA/QC and in-process quality assurance. The Executive, IPQA role in Ahmedabad is ideal for MSc Chemistry Jobs and professionals looking to grow in pharmaceutical QA/QC careers. The company emphasizes compliance, safety, and professional development in a structured manufacturing and laboratory environment.
- Job Post: Executive, IPQA
- Location: Ahmedabad, Gujarat, India
- Job Identification: 6934
Job Description
To ensure compliance with cGMP and regulatory requirements by performing in-process quality assurance activities on the manufacturing and packaging shop floor, including real-time monitoring, documentation review, sampling, and area/machine clearances, thereby supporting the production of high-quality injectable pharmaceutical products.
Essential Functions:
- To ensure cGMP and compliance review on the manufacturing shop floor and packing areas.
- Supervision of dispensing activities on the manufacturing shop floor and in packing areas.
- Responsible for providing machine and area clearance.
- Conduct Sampling of reserve sample, stability sample, and In-process checks during execution of batch packaging as per the Batch Packaging Record (BPR) and stability study protocol.
- Online Documents and records review, like equipment’s logbooks, dispensing logs, housekeeping records, environmental monitoring records, calibration records, batch records, etc.
- Calibration of IPQA instruments.
- Review of and release of Batch.
- Responsible for reviewing the in-process and finished product COA before batch release.
- Perform aseptic behaviour/practice monitoring of operators (while working inside the aseptic process area) during each aseptic batch (GMP batch) as per SOP.
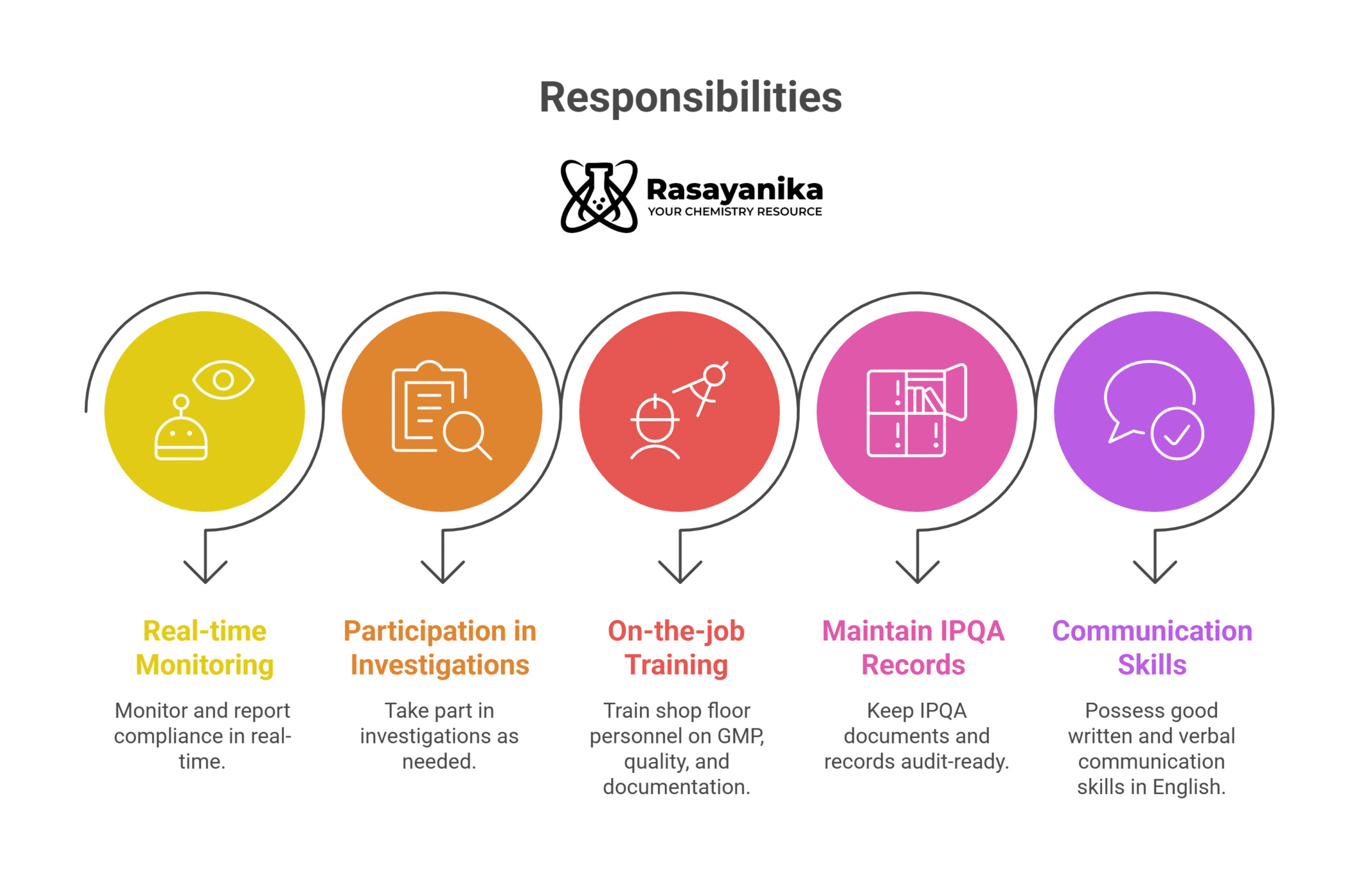
Additional Responsibilities:
- Real-time Monitoring and Compliance Reporting.
- Participation in Investigations.
- Provide on-the-job training to shop floor personnel on GMP practices, quality standards, and documentation practices.
- Maintain IPQA-related documents and records in an audit-ready state.
- To have good written and verbal communication skills in the English language.
Skills:
- In-Process Quality Control (IPQC) – Mastery
- Compliance and Regulatory Knowledge – Advanced
- Aseptic Process Monitoring & Compliance – Advanced
- Line Clearance & Batch Record Review – Intermediate
- Deviation, Investigation & CAPA Management – Advanced
- In-Process Checks & Monitoring – Mastery
- Deviation & Change Control Management – Advanced
- Equipment & Facility knowledge – Advanced
- Investigation Skills for Root Cause Analysis – Advanced
- Analytical Quality Assurance – Advanced
Qualifications
- Education: B.Pharm / M.Sc / M.Pharm
- Experience: 2 – 4 Years