Drug Discovery in India: Step-by-Step Chemistry Involved & Job Prospects
What is Drug Discovery?
The drug discovery process is considered one of the most meticulous processes through which chemical compounds are designed and products are developed. With the help of this process, researchers are capable of manufacturing drugs to treat and target diseases.
And over the past years, there has been a significant evolution in the process of drug development. And this not only strengthens India’s pharmaceutical market but also impacts global career trends and research & development processes.
As technology advances, the process of developing a drug is also advancing, and we can observe that artifical intelligence (AI) is incorporated in these processes as well to boost efficiency as well as reduce manufacturing time. With computational genomics and cheminformatics, one can efficiently screen molecules and identify promising drug candidates.
In this article, let us explore the different stages of drug discovery and understand the job prospects in drug development.
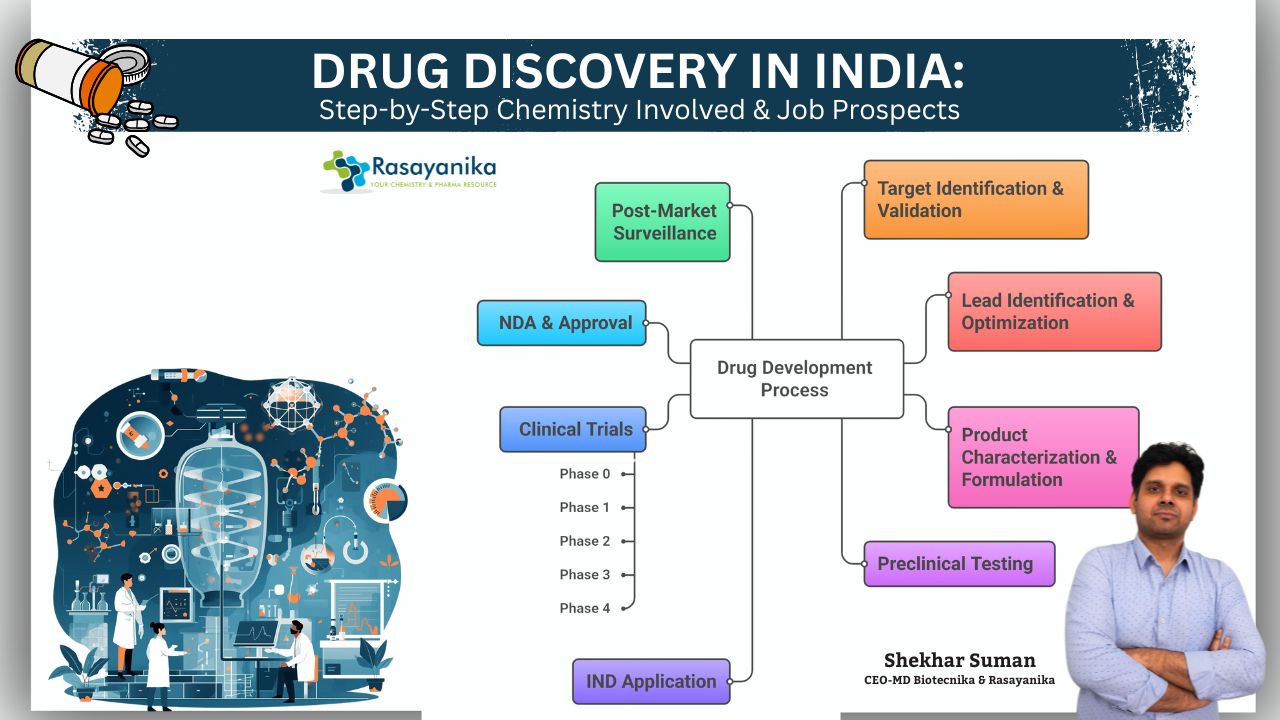
The Step-by-Step Chemistry Behind Drug Discovery
At the heart of drug innovation is the detailed and deliberate drug discovery chemistry process. Here’s how it unfolds in the Indian context:
1. Target Identification
To manufacture a drug, we first need to understand the cause of the disease and where and how to target it to cure that particular disease. Understanding the biomolecule to be targeted is a highly crucial step. That bimolecule can be a protein, an enzyme, or a gene that needs to be targeted. Researchers then select this molecule as a potential drug target.
Example: In diabetes, insulin receptors are the primary targets.
To successfully complete this first step, researchers use various techniques such as bioinformatics, genetic studies, protein expression analysis, and cell-based studies.
2. Target Validation
Before advancing to the second step of drug discovery, one must have explicit confirmation regarding the target found in the previous step. Scientists usually confirm the targets identified and will validate that the particular biomolecule is linked to the disease, and then will proceed to the next step. This step is achieved through lab experiments and model systems, such as
- Turning the gene “on” or “off” using CRISPR or siRNA.
- Observing how this affects disease symptoms in cells or animals.
- Checking whether blocking this target helps improve the condition.
If the results are consistent, the target is considered validated.
3. Lead Identification
In the process of drug discovery, one crucial step is identifying a lead chemical compound that interacts with the target.
Using techniques such as high-throughput screening, scientists test thousands of molecules to identify which show a beneficial effect; these are called “hits.”
Among these, only a few are further refined into “leads”, drug-like compounds with some desired activity. An ideal lead candidate should be stable and safe. It should also possess a strong binding site to the target molecule. One of the significant criteria for a lead candidate is being easily synthesizable. They should have drug-like properties as well.
4. Lead Optimization
This is where chemists play a crucial role. The lead compound makes it safer, stronger, and more effective. Through several rounds of synthesis and testing, they modify the chemical structure to improve the lead candidates’ target-binding sites, which should also have good absorption and metabolism. Optimizing the lead candidate also involves creating a safety profile that has lower toxicity.
They use tools like mass spectrometry, NMR screening, and computer modeling to predict how the molecule behaves in the body.
At the end, one or two optimized drug candidates move forward for animal testing.
5. Product Characterization
Once a potential drug looks promising, scientists study its physical and chemical properties, such as:
- Molecular structure and weight
- Stability, solubility, and pH
- Toxicity and biological activity
These tests ensure the molecule can be safely developed into a medicine.
6. Formulation and Development
Once the product characterization is achieved, the drug formulation and development will start. The drug can be given in different formats, such as a tablet, capsule, injection, or in other forms.
Here, pharmaceutical scientists are required to formulate the solubility as well as the dissolution rates of the drug being developed. The stability of the drug is also an important factor and should be properly stable. One of the challenges is to make sure that the drug gets released slowly inside the body, and the drug compound and its effects stay longer inside.
7. Preclinical Testing – Testing in Animals
Before human trials, the drug must be tested in laboratory animals to assess its safety and efficacy. Here, we can observe researchers perform two main types of studies:
- Pharmacology studies: How the drug behaves (ADME – absorption, distribution, metabolism, excretion).
- Toxicology studies: Checking for harmful effects or organ damage.
These tests reveal the safe dosage range and help predict how the drug might act in humans.
If successful, researchers apply for permission to test in humans through an Investigational New Drug (IND) application.
8. Investigational New Drug (IND) Application
To begin clinical trials, scientists must submit an IND to regulatory authorities (like the FDA).
The application includes:
- Preclinical data and animal study results
- How the drug will be made
- Protocols for planned human studies
- Information about the investigators
Only after approval can human trials begin.
9. Clinical Trials – Testing in Humans
Clinical research takes place in four phases, each designed to answer specific questions:
Phase 0 – Microdosing
- This step includes 10 – 15 volunteers, and a minimal amount of the dose is tested on them.
- Checks how the drug moves in the body (pharmacokinetics)
- Helps decide whether to continue development
Phase 1 – Safety & Dosage
- In this step, approximately 20–80 healthy volunteers are involved to test the drug.
- Determines the safe dose and short-term side effects
- After this step, about 70% of drugs move to Phase 2
Phase 2 – Effectiveness & Side Effects
- Here, the number of participants is increased further. A small population of 100–300 patients with the disease is tested with the drug molecule.
- Evaluates whether the drug actually works and continues safety monitoring
- About 33% of drugs move to Phase 3
Phase 3 – Large-Scale Confirmation
- This particular step consists of more than 1,000–3,000 participants
- Confirms effectiveness and identifies rare or long-term side effects
- Successful drugs proceed to market approval.
Phase 4 – Post-Market Surveillance
- Conducted after the drug is launched
- Monitors real-world safety and performance
- Detects rare adverse effects over time
10. New Drug Application (NDA) & Approval
After all trial phases, the company compiles all results into a New Drug Application (NDA) and submits it to the regulatory body.
The NDA contains:
- Curated data from all the tests done in the previous steps, which included tests on animals as well as human results.
- The NDA will have detailed information on the manufacturing details
- Labeling and dosage instructions
- Safety updates and proposed warnings
Regulators carefully review this data. This particular step alone can take 6 to 10 months. If approved, the drug can finally be sold in the market.
11. Post-Market Surveillance
Even after approval, monitoring does not stop. The drugs are closely monitored post-marketing as well. Doctors, patients, and regulatory bodies report any new side effects. The information helps refine dosage, update warnings, or even withdraw unsafe drugs.
| Stage | Purpose | Main Activities |
| Target Identification | Find disease-related molecule | Bioinformatics, genetic studies |
| Target Validation | Confirm its role in the disease | Gene editing, pathway studies |
| Lead Identification | Find potential active molecules | Screening thousands of compounds |
| Lead Optimization | Improve structure & safety | Chemical modification, modeling |
| Product Characterization | Understand the molecule fully | Analyze properties and behavior |
| Formulation | Create a dosage form | Tablet, capsule, injection design |
| Preclinical Testing | Test in animals | Pharmacology, toxicology |
| IND | Apply for human trials | Submit preclinical data |
| Clinical Trials | Test in humans | 4 phases: 0–IV |
| NDA | Regulatory review | Submit full documentation |
| Post-Market | Ongoing monitoring | Safety and risk management |
Notable Success Stories
Indian pharma companies have made notable advances:
- Synariam is a new malaria drug developed by Ranbaxy Laboratories.
- Lipaglyn is India’s first new chemical entity, which was developed by Cadila Healthcare and is used for managing diabetes.
Emerging Career Opportunities
With the pharmaceutical sector expected to cross USD 130 billion by 2030, India offers multiple job opportunities for scientists, engineers, marketers, and digital experts.
- Research & Development
R&D remains one of the major sectors hiring candidates interested in the drug discovery process. The sector provides suitable opportunities for specialists in formulation, bioinformaticians, as well as AI specialists. With the help of digital transformation, one can effectively predict the analytics.
- Manufacturing & Process Chemistry
Roles for production chemists, process engineers, and quality assurance professionals are expanding due to India’s global leadership in manufacturing and process innovation.
- Clinical Trials & Regulatory Affairs
Careers in clinical data management, pharmacovigilance, regulatory affairs, and quality compliance are surging as India’s dominance in trials and global exports grows.
- Entrepreneurship & Startups
The rapid emergence of biotech and health-tech startups fosters entrepreneurial roles in telemedicine, digital pharmacology, and green chemistry.
With the advent of science and technology, India is at the forefront of manufacturing drugs and thereby expanding the opportunities to work in this sector. The job market is increasing, and AI is enabling faster drug development. For all these technological advancements, one should have a thorough subject knowledge, and chemistry helps in gaining that technological knowledge. The country is reshaping global pharmaceutical paradigms and fueling the next wave of career and research opportunities.