Executive QA Jobs at Enzene Biosciences | MSc Chemistry Graduates Apply Now
Are you looking for an exciting opportunity to build a strong career in pharmaceutical quality assurance? This role offers excellent Executive jobs in Pune for professionals seeking QA and chemistry roles within a cGMP-regulated Drug Substance manufacturing environment. Ideal for M.Sc. Chemistry and M.Pharm graduates, this position provides hands-on exposure to IPQA operations, shop-floor compliance, batch record review, and quality systems, making it a valuable opportunity for candidates aiming to grow into pharmaceutical QA executive roles.
About Enzene Biosciences
The company is a leading pharmaceutical organization with a strong presence in Drug Substance manufacturing and a commitment to global quality standards. It operates under strict cGMP, QMS, and regulatory compliance frameworks, catering to regulated markets such as USFDA, EMA, and WHO. With a focus on innovation, quality excellence, and continuous improvement, the organization provides a robust platform for professionals seeking QA jobs, chemistry jobs in Pune, and executive-level opportunities. The Quality Assurance function plays a critical role in ensuring product integrity, regulatory readiness, and operational excellence.
- Job Title: Executive / Senior Executive – QA (DSQA)
- Location: Pune, Maharashtra, India
- Experience: 2–9 Years
- Qualification: M.Sc / M.Pharm
Job Purpose
To ensure cGMP compliance
at the shop floor for Drug Substance (DS) manufacturing through effective line clearance, documentation review, in-process oversight, and quality systems activities.Key Responsibilities
- Perform manufacturing line clearance on the shop floor for DS activities.
- Ensure proper execution and compliance of in-process checks during manufacturing operations.
- Conduct routine GMP rounds at the Drug Substance facility and document observations.
- Review online batch manufacturing records (BMR) for accuracy, completeness, and compliance.
- Review and approve process validation protocols and reports, cleaning validation protocols and reports.
- Review and manage Standard Operating Procedures (SOPs) and other protocols/reports related to shop floor operations.
- Coordinate collection and submission of samples as per BMR, specifications, process validation, and stability protocols.
- Perform regular shop floor compliance rounds to ensure adherence to cGMP and follow up on corrective actions.
- Prepare and support Annual Product Quality Review (APQR/PQR) documents.
- Initiate and manage Change Control, Deviations, Incidents, and CAPA, including investigation support and timely closure.
- Participate in QMS investigations and root cause analysis.
- Ensure continuous cGMP compliance and regulatory readiness at the shop floor.
- Perform risk assessments for critical manufacturing and quality processes.
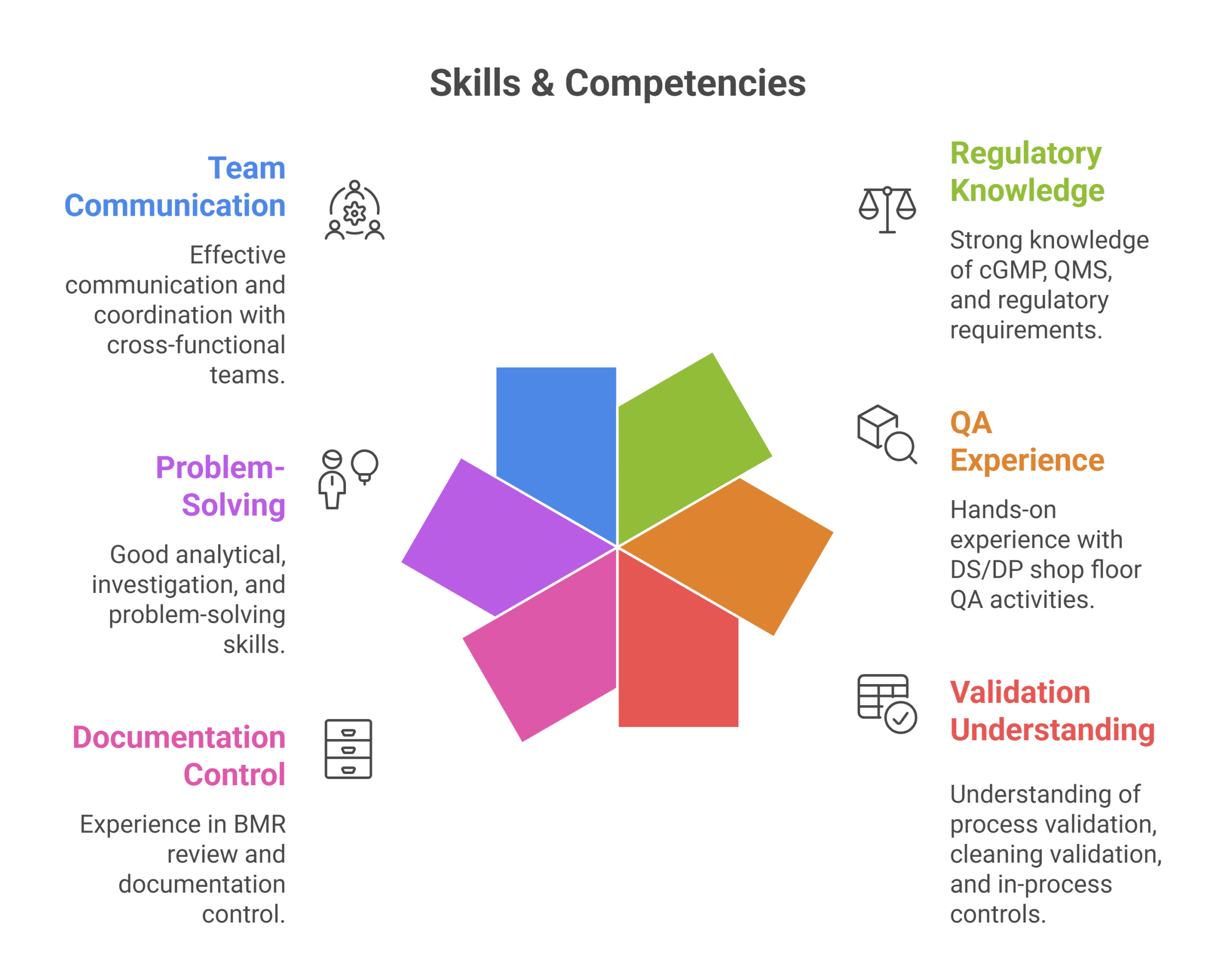
Skills & Competencies
- Strong knowledge of cGMP, QMS, and regulatory requirements
- Hands-on experience with DS/DP shop floor QA activities
- Understanding of process validation, cleaning validation, and in-process controls
- Experience in BMR review and documentation control
- Good analytical, investigation, and problem-solving skills
- Effective communication and coordination with cross-functional teams
Regulatory Exposure
USFDA, EMA, WHO, and other global regulatory guidelines (preferred).