MSc Chemistry Jobs at Syngene | Chemistry Candidates Apply
Are you looking for MSc Chemistry Jobs that combine leadership, innovation, and scientific excellence? Syngene is inviting experienced professionals to join its leadership team as a Lead – Technical Training in Bengaluru. This role offers a unique opportunity to shape advanced learning programs, support scientific talent, and grow your career with one of the most respected names in Syngene Careers.
About Syngene
Syngene International Ltd. is a leading contract research, development, and manufacturing organization providing integrated scientific services across discovery, development, and commercial manufacturing. Known for its strong research culture and global collaborations, Syngene Jobs offers excellent opportunities for professionals seeking long-term growth and impactful careers in science. If you are exploring MSc Chemistry Jobs, Syngene Careers provides a platform to innovate, lead, and make a difference.
- Division: Essential Functions
- Position Title: Lead– Technical Training
- Job Level: Thought Leader
- Location: Bengaluru, India
About the Role:
Syngene is seeking a seasoned professional to lead its Technical Training function, responsible for building and scaling learning programs that strengthen the scientific and technical capabilities of our workforce. This leadership role is pivotal in ensuring our teams across R&D, manufacturing, and analytical services are equipped
with the latest skills and knowledge to meet evolving client expectations and regulatory standards.
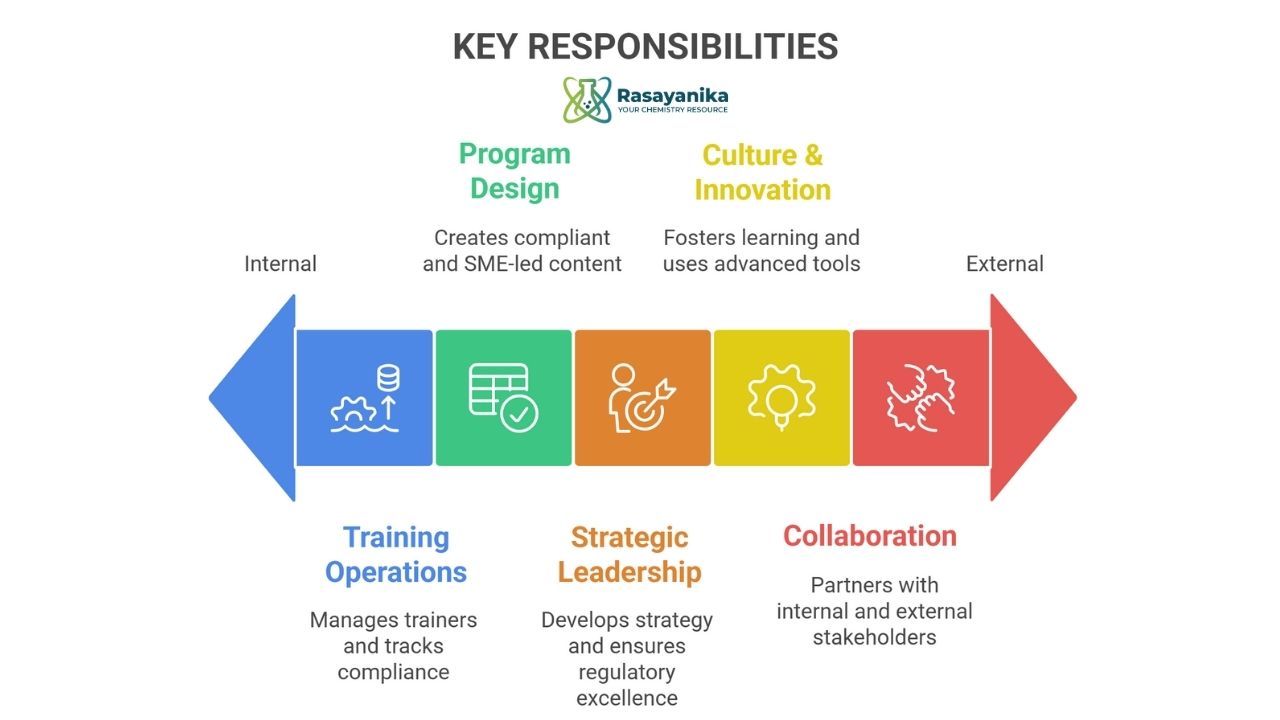
Key Responsibilities
1. Strategic Leadership
- Define and implement a forward-looking technical training strategy aligned with Syngene’s business goals and industry developments.
- Partner with senior leaders to assess skill gaps and identify training needs across departments, including biologics, chemistry, manufacturing, and analytics.
- Champion initiatives that elevate technical proficiency in areas such as drug discovery, process development, cGMP manufacturing, and regulatory compliance.
2. Program Design & Delivery
- Lead the development of engaging and effective training programs—ranging from e-learning modules to hands-on workshops and certification courses—tailored to scientific roles.
- Ensure training content reflects the latest advancements in biopharmaceutical technologies and complies with global regulatory standards (e.g., FDA, EMA).
- Collaborate with internal subject matter experts to design specialized modules in areas such as protein characterization, nitrosamine testing, and biologics manufacturing.
3. Training Operations
- Manage a team of trainers and learning professionals to deliver consistent, high-quality training across Syngene’s Bangalore facilities.
- Oversee the deployment and optimization of learning management systems (LMS) to track progress, certifications, and compliance.
- Establish metrics to evaluate training effectiveness and drive continuous improvement through data-driven insights.
4. Cross-Functional Collaboration
- Work closely with teams in R&D, manufacturing, quality assurance, and regulatory affairs to ensure training supports operational excellence and client deliverables.
- Build strategic partnerships with external training providers, academic institutions, and industry bodies to enrich Syngene’s learning ecosystem.
- Support global client engagements by ensuring technical teams are trained to meet international standards and expectations.
5. Culture & Innovation
- Foster a culture of continuous learning and knowledge sharing across Syngene’s scientific community.
- Introduce modern training methodologies, including virtual reality simulations and AI-enabled learning tools, to enhance learner engagement and outcomes.
- Align all training initiatives with Syngene’s core values—integrity, excellence, and professionalism.
Qualifications & Experience:
Education
- Master’s or Ph.D. in Biotechnology, Chemistry, Pharmaceutical Sciences, or a related scientific discipline.
- Additional certifications in Learning & Development, Instructional Design, or Business Administration are advantageous.
Experience
- Should have experience in technical training, learning & development, or talent development.
- Demonstrated success in designing and delivering technical training programs within the biopharmaceutical or contract research industry.
- Familiarity with global regulatory standards (e.g., cGMP, GLP, GCLP) and experience working with international clients.
Skills & Competencies
- Strong understanding of biopharmaceutical processes, including drug discovery, development, and manufacturing.
- Expertise in instructional design, adult learning principles, and modern training technologies (e.g., LMS, e-learning platforms).
- Proven leadership and team management capabilities, with the ability to inspire and guide diverse teams.
- Excellent communication and stakeholder engagement skills.
- Analytical mindset with a focus on measuring impact and driving continuous improvement.