Top QA QC Testing Tools Every Pharma Student Should Know in 2025
What if the drug you took while you were ill depended on a Researcher or Scientist’s detective skill sets? Visualize a Pharmaceutical Scientist as that very detective. This professional takes up each vial or culture as a mystery case to solve, every Chemical titration curve a hidden clue to be found, as well as every Microbial test a silent witness or evidence. But do you know what is at stake? It’s human lives and their well-being. So, what’s the verdict? It is an effective and safe medication.
Pharmaceutical Quality Control professionals act as the Sherlock Holmes of the Pharmaceutical industry. But without the proper tools, specific critical details could be overlooked. Due to this mishap, significant errors can occur, which could eventually compromise patient health and safety. With skilled QA & QC professionals, each injection, Biologic, vaccine, or tablet meets the quality Regulatory standards of reliability, accuracy, as well as purity. QA & QC aren’t just departments, they’re the foundation of Drug Safety worldwide.
For aspiring professionals and enthusiastic students, mastering and understanding QA QC Testing Tools is the key to building a successful career in the Pharmaceutical industry. In this article, some critical QA (Quality Assurance) & QC (Quality Control) Testing Tools are highlighted, which every Pharmaceutical student or professional must know. The importance of these tools in the Pharmaceutical and Life Sciences industry is also explained, along with recent trends and the advantages of utilizing them to gain a competitive edge over others. Their importance in the Pharmaceutical and Life Sciences industry
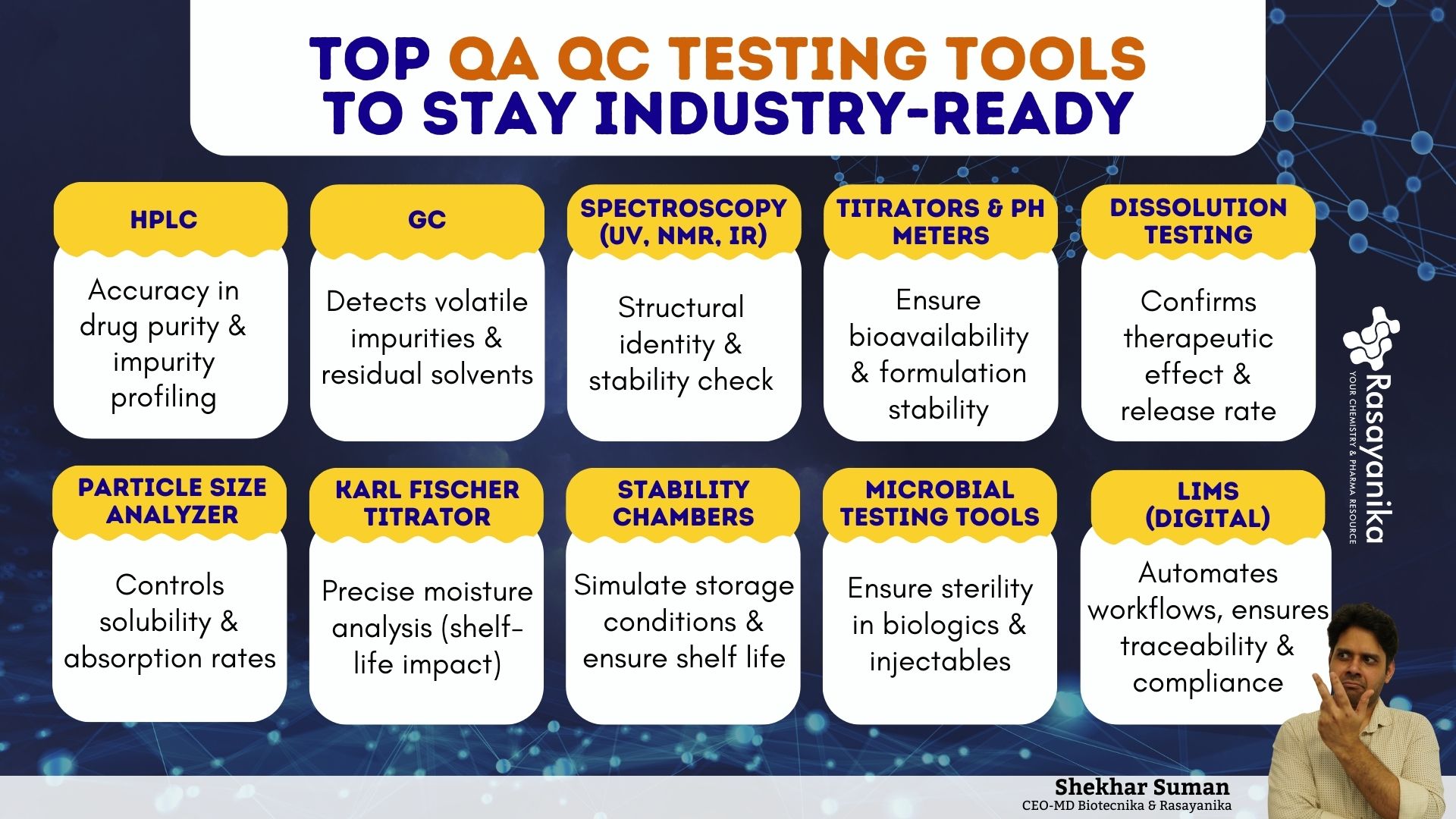
01 High-Performance Liquid Chromatography (HPLC)
HPLC is widely used in Pharmaceutical Quality testing. It is used to identify, quantify, and separate the components of complex mixtures and products. It is one of the most trusted characterization techniques due to its reproducibility and unmatched accuracy. From APIs (Active Pharmaceutical Ingredients) to degradation products and impurities, HPLC provides the precision necessary to ensure that drugs and products are both effective, safe, and of satisfactory quality for patients worldwide. Hands-on exposure to QA QC Testing tools, such as HPLC, builds a strong foundation for a Pharmaceutical career.
- Why it matters: Ensures that the purity, Regulatory compliance, quality, as well as safety of drugs and products.
- Relevance today: Paramount for Biologics, Regulatory submissions, as well as Generics domains.
- Student advantage: Understanding and hands-on HPLC training is highly valued and sought after by top companies; nearly every QC laboratory depends upon it these days.
- Trend outlook: Advancements in UHPLC (Ultra-High-Performance Liquid Chromatography) are improving sensitivity and decreasing analysis times.
02 Gas Chromatography (GC)
GC is one of the powerful tools employed for Pharmaceutical analysis, especially for analyzing and separating volatile compounds from a product. In QC testing, it plays a crucial role in ensuring drug safety by detecting residual solvents, impurities, as well as other trace components of the product that could affect the safety or efficacy of a Pharmaceutical formulation and product.
- Why it matters: Detects impurities and toxic components that could compromise the quality and safety of drugs and products.
- Relevance today: Compulsory in impurity profiling and stability studies.
- Student Advantage: GC expertise prepares professionals for industry-ready and Quality Control career roles in R&D (Research & Development), QC, as well as QA.
- Trend outlook: Coupling GC with MS (Mass Spectrometry), i.e., GC-MS (Gas Chromatography-Mass Spectrometry), enhances sensitivity and trace analysis.
A degree alone doesn’t equate job-ready skills
Do not stop at a degree, add skills that industries want
✔️Learn QC-LC-MS with Biotecnika’s Industrial Training Program
Get to work on projects, get placement assistance & much more
Want to Enrol? Click here
03 Spectroscopy Techniques (UV, NMR, & IR)
Spectroscopy Techniques form the backbone of Pharmaceutical analyses by enabling Scientists and Researchers to study how matter/compounds interact with various types of radiation, such as electromagnetic or light.
UV (Ultraviolet) Spectroscopy is widely utilized for purity testing and drug quantification. NMR (Nuclear Magnetic Resonance) Spectroscopy provides a detailed and in-depth interpretation of complex Chemical compounds. IR (Infrared) Spectroscopy is used to verify molecular structures and identify functional groups. Together, these Spectroscopy Techniques provide essential insights into drug stability, formulation quality, as well as identity. This makes them quite essential in Pharmaceutical QC as well as R&D.
- Each technique offers a different advantage:
- UV Spectroscopy: Quantifies the concentration of Drug compounds.
- NMR: Reveals detailed structural information of drugs and products.
- IR Spectroscopy: Identifies Chemical bonds & functional groups of compounds.
- Why it matters: It confirms the structural integrity and identity of drugs and products, which are an essential part of Quality Assurance.
- Relevance today: It is a Regulatory must-have Technique for the Pharmaceutical industry.
- Student advantage: Connects Chemistry knowledge with real-world Pharmaceutical testing.
- Trend outlook: Recently, Portable Spectrometers have been enhancing testing faster, cheaper, and more accessible. These are transforming the way QA QC Testing Tools are applied in real-world Pharmaceutical laboratories.
04 Titrators and pH Meters
Titration and pH (potential of Hydrogen) measurement are one of the most fundamental techniques utilized in the Pharmaceutical QC. It showcases a drug’s solubility, overall bioavailability, and stability, which makes these techniques critical for safe and consistent drug formulations in industrial R&D.
- Why it matters: Accurate titration and pH monitoring ensure Drug formulations meet Pharmacopeial requirements and safeguard product stability according to the norms and Regulations.
- Relevance today: In Biologics, oral drugs, and injectables, maintaining pH within a narrow range is vital for both therapeutic effectiveness as well as safety of the drug.
- Student advantage: Hands-on training with titrators and pH meters is one of the first and most basic skills that Pharmaceutical students must demonstrate, and QC recruiters look for in an ideal candidate.
- Trend outlook: Modern automated systems and digital titrators with integrated data logging are improving efficiency as well as streamlining compliance.
05 Dissolution Testing Apparatus
The Dissolution Testing Apparatus is one of the most critical QC instruments in the Pharmaceutical sector. It measures the rate and efficiency at which an API (Active Pharmaceutical Ingredient) is released from a dosage form (such as a capsule or tablet) into a liquid medium simulating conditions within the human GIT (Gastrointestinal Tract). This technique ensures that the drug delivers the intended therapeutic effect when administered to the patient. This makes it one of the essential QA QC Testing Tools for generic Drug formulations.
- Why it matters: Ensures consistent and effective drug absorption and release in the body.
- Relevance today: Mandatory for controlled and generic-release drug formulations in the market.
- Student advantage: Familiarity with this technique strengthens expertise in Pharmacokinetics and gives an upper hand in the Pharmaceutical settings.
- Trend outlook: Real-time monitoring and automation are making tests more efficient and reliable.
06 Particle Size Analyzers
A particle size analyzer is one of the fundamental testers for quality parameters in the Pharmaceutical industry. It directly influences the drug dissolution rate, solubility, overall bioavailability, as well as absorption. Even the smallest variations can alter therapeutic stability or outcomes. This makes this technique important across suspensions, emulsions, advanced nanomedicines, as well as solid dosage forms. Nanoscale analysis is now a crucial component of modern QA QC Testing Tools for advanced drug formulations.
- Why it matters: Controls formulation performance and ensures uniformity.
- Relevance today: Especially important for inhalation drugs, injectables, and nanomedicines.
- Student advantage: Skills here open career paths in formulation development and QC.
- Trend outlook: Nanoscale particle analyzers are in demand for advanced drug delivery systems.
07 Karl Fischer Titrator
Moisture content is a CQA (Critical Quality Attribute) in the Pharmaceutical industry. It is essential, as even trace amounts of water can impact drug potency, stability, shelf life, as well as crystallinity. The Karl Fischer (KF) titrator remains the gold standard for determining precise water content in drug excipients, raw materials, and finished formulations in the industry.
- Why it matters: Excess moisture can degrade the safety and stability of a drug or product.
- Relevance today: A regulatory requirement for nearly all dosage forms.
- Student advantage: Expertise demonstrates readiness for core QC processes.
- Trend outlook: Modern instruments integrate data logging and automation for compliance.
08 Stability Chambers
Drug products must remain safe, effective, and chemically stable throughout their shelf life, regardless of where they are distributed. Stability chambers are specialized controlled environments designed to simulate temperature, humidity, and light conditions that mimic real-world and accelerated storage scenarios. They play a pivotal role in stability studies, which are a regulatory requirement for the approval and commercialization of drugs. This technique is mandatory for Pharmaceutical companies entering global markets.
- Why it matters: Ensures safety and efficacy throughout the product lifecycle.
- Relevance today: Central to regulatory submissions for global markets.
- Student advantage: Practical knowledge aligns with industry protocols.
- Trend outlook: Energy-efficient, IoT-enabled chambers improve monitoring and reliability.
09 Microbial Testing Tools
Pharmaceutical products should be free from harmful microorganisms to ensure Regulatory compliance as well as patient safety. Tools such as Laminar Airflow (LAF) cabinets, autoclaves, microbial detection kits, endotoxin testing systems, as well as Biosafety Cabinets (BSCs) are indispensable in maintaining sterility. Rapid microbial detection, combined with QC software testing tools, is revolutionizing sterility checks in the Pharmaceutical industry globally.
- Why it matters: Detects microbial contamination in Biologics, sterile drugs, and injectables.
- Relevance today: Essential in advanced therapy products, biologics, as well as vaccines.
- Student advantage: Microbiology skills expand employability in QC & QA roles.
- Trend outlook: Rapid microbial detection tools are replacing time-consuming culture methods globally.
10 LIMS (Laboratory Information Management Systems )
In today’s Pharmaceutical industry, compliance, efficiency, and data integrity are non-negotiable globally. LIMS is a digital backbone that automates data capture, integrates laboratory workflows, facilitates Regulatory compliance, as well as ensures traceability. It acts as a central hub where audit trails, sample management, testing results, as well as inventory are seamlessly monitored, making it a digital pillar among QA QC Testing Tools.
- Why it matters: Ensures traceability, streamlines audits, as well as manages data.
- Relevance today: An essential tool for knowledge of compliance with global Regulatory authorities.
- Student advantage: Knowledge of LIMS demonstrates digital readiness worldwide.
- Trend outlook: Cloud-based LIMS integrated with AI is driving the development of “smart labs.”
How to Get Started as a Student
- Stay Updated: Follow the guidelines of the EMA (European Medicines Agency), WHO (World Health Organization), and FDA (Food and Drug Administration) to understand the global Regulatory importance.
- Certifications: Enroll in QA QC Testing Tools programs to validate your skills and expand your horizon.
- Leverage Technology: Gain knowledge on the basics of digital tools such as automated Chromatography systems and LIMS.
- Hands-on Training: Seek workshops and internships where you can practice these tools and gain expertise.
Why Students Must Stay Ahead?
Pharmaceutical Quality Testing is no longer limited just to traditional laboratories, but it is now shaped by digital tools, global Regulations, as well as automation. Top employers seek skilled candidates who can blend conventional laboratory expertise with data-driven and modern approaches in the Pharmaceutical industry.
For students, this translates into three significant advantages:
- Global Readiness: With Pharmaceutical hubs expanding in regions such as Europe, the US, and Asia, tool expertise makes you globally ready for the Pharmaceutical industry.
- Enhanced Employability: Hands-on skills with these tools make you a priority candidate for QA&QC Testing roles in the Pharmaceutical industry.
- Future-Proof Careers: As digital Pharmaceutical and AI-driven QC tools expand, a strong foundation ensures adaptability and increased efficiency for next-generation innovations globally.
Mastering Pharmaceutical Quality testing tools is becoming a career accelerator in the Pharmaceutical industry. Top techniques such as GC, HPLC, Spectroscopy, as well as FTIR, are key to drug safety. Emerging and advanced technologies, such as combination MS and portable Spectrometers, are shaping the industry’s future.
For professionals and graduates, knowledge of these tools and techniques offers more than just technical understanding. It provides adaptability to innovation, a competitive edge in a demanding industry, as well as global career readiness.
In today’s world, every drug product is scrutinized for its efficiency, quality, and safety. Hence, expertise in QA/QC Testing Tools is your passport to success in the Pharmaceutical Industry.