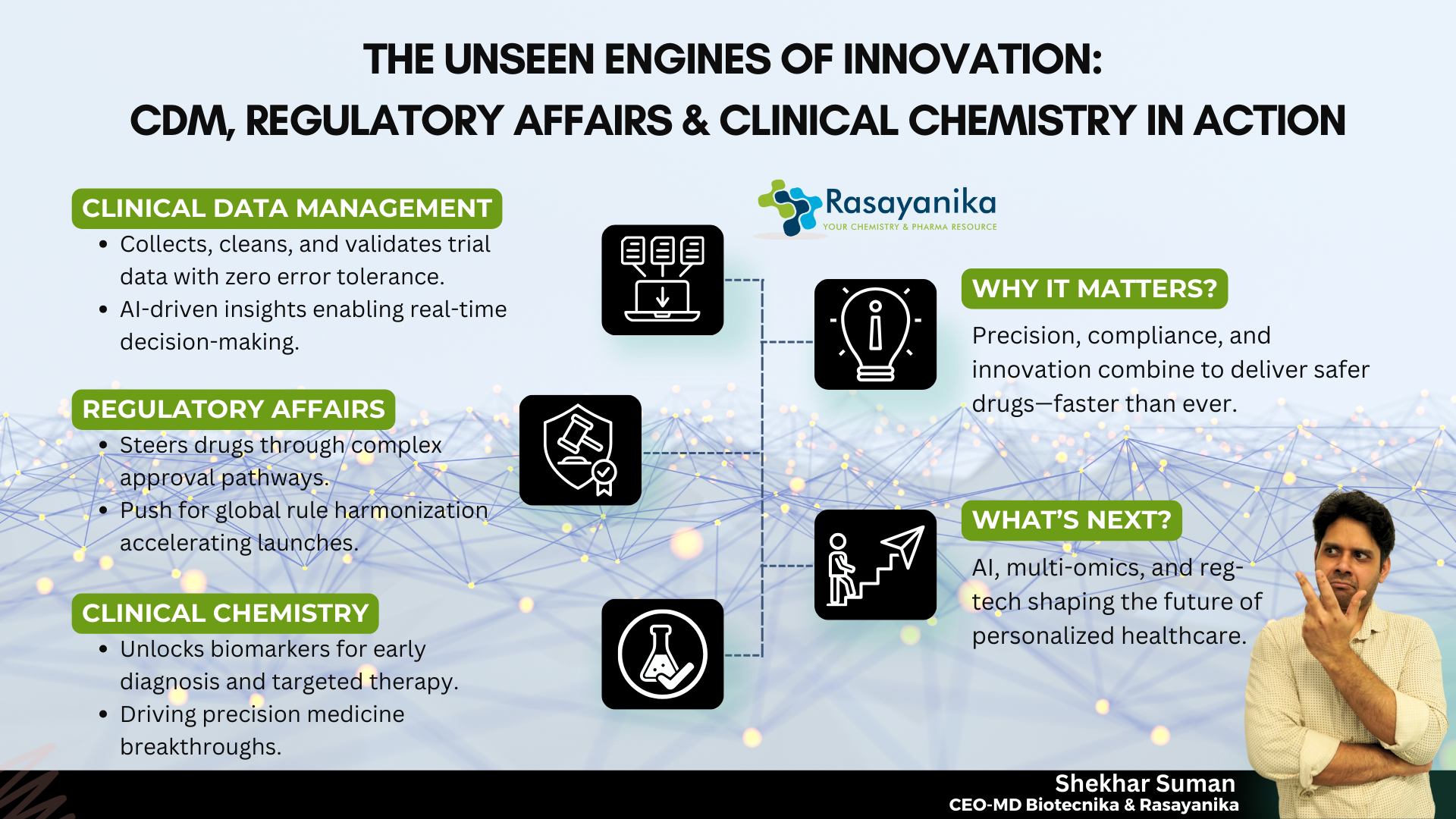
CDM, Regulatory Affairs & Clinical Chemistry: The Triad Reshaping Drug Discovery
In the year 2020, the whole world was ailing and anxiously waiting for a powerful vaccine or treatment that could wipe out COVID-19. That time, the real heroes were the professionals in their white coats, such as healthcare workers and Scientists. But do you know who the professionals were behind the scenes? They were the Clinical Data Managers, Clinical Chemists, as well as Regulatory Strategists. The Clinical Data Managers supervised the entire Trial process and checked every minute detail for accuracy in treatment and medications. Clinical Chemists work day and night, experimenting and testing after testing to find promising medicine innovations that are safe and effective for people worldwide. Regulatory Strategists navigated the complicated labyrinth of Global approvals and worked hand in hand to deliver agility without risking the safety and effectiveness of the medication and treatments.
Together, this incredible triad of CDM, Regulatory Affairs & Chemistry has revolutionized the world in times of need and beyond. They are the backbone of a new age in Drug Discovery, and are more intelligent, trustworthy, as well as faster in action.
In this rapidly evolving world of Scientific advancements, Drug Discovery
is giving way to a more data-driven, regulatory-compliant, and agile ecosystem, powered by CDM, Regulatory Affairs & Chemistry working in unison.. At the heart of this revolution is the powerful triad of CDM, Clinical Chemistry, as well as RA. These are the three central pillars reshaping therapies, innovation, development, as well as delivery.The Changing Face of Drug Discovery
As global Health challenges become increasingly complex, there is an urgent need for safer, more precise, as well as faster Drug Development pathways. Technological advancements, such as AI (Artificial Intelligence), ML (Machine Learning), wearable health monitors, RWE (Real-World Evidence), as well as multi-Omics platforms, are becoming essential and integral to the Pharmaceutical world.
In this new era of Innovation, RA, Clinical Chemistry, as well as CDM no longer function as individual departments; instead, they come together as a strategic Triad. They ensure that strong Clinical validation, clear Regulatory pathways, and quality Data support Drug processes.
In this era of Technological advancement, where Data and Machines are considered the new Drugs, CDM, Regulatory Affairs & Chemistry form the foundation of Clinical Research and Development strategies.
-
What is it?
Clinical Data Management or CDM is basically the management of Clinical Trial Data to ensure that it is statistically safe and sound, as well as reliable. CDM professionals apply rigorous and strict Validation checks, work closely with Clinical teams to minimize missing information and errors, and design Data collection systems (through Case Report Forms or CRFs).
-
CDM Professional’s Role
CDM involves the planning, collection, maintenance, validation, as well as cleaning of Data generated during the Clinical Trials process. Their primary goal is to ensure that the Data used for Statistical analysis and Regulatory submission is verifiable, complete, as well as accurate.
But today’s CDM professionals go beyond spreadsheets. With the rise of ePROs (electronic Patient-Reported Outcomes), cloud-based EDC (Electronic Data Capture) systems, and DCTs (Decentralized Clinical Trials), Data Managers navigate the complex digital landscape.
-
Why It Matters?
- Patient safety: Cleaning of Data that impacts the safety and efficacy analysis of investigational Drugs in the industry.
- Decision-making: Data that is reliable enables informed and accurate decision-making for Clinical Trials processes
- Regulatory approval: Global Regulatory Frameworks and authorities seek and demand error-free and audit-ready Data for regulated work.
-
Recent Trends in CDM:
- eSource data capture and Remote monitoring
- Digital Health platforms and wearables for accessing continuous patient data collection and analysis.
- Integration of automation and AI for real-time Health Data checks and analyses.
-
Deep Insights on CDM
- AI & Automation: AI has been increasingly utilized for discrepancy resolution, data trend prediction, as well as auto-query generation. Thereby, it helps reduce human error and manual review times.
- Patient-Centric Data: From remote sensors to wearables, real-world data is supplementing conventional Clinical Trial endpoints. This provides enhanced information on patient experiences with the Medical scenarios.
- RBM (Risk-Based Monitoring): It is a real-time Data Analytics approach that prioritizes site visits and focuses resources on anomalies, thereby enhancing both trial efficiency and data integrity.
CDM professionals are emerging Data Architects, shaping safety profiles, therapeutic success, as well as Regulatory decisions worldwide.
Regulatory Affairs (RA) is a Compliance-driven domain that also serves as a strategic partner in Drug Development processes. A potential Drug’s approval and success depend on its efficacy and safety, but also on how it can meet global Regulatory expectations.
-
What is it?
RA ensures that drugs and compounds are tested, developed, produced, and marketed in Compliance with domestic and Global Regulations. These professionals collaborate with Regulatory Authorities such as the EMA (European Medicines Agency) in Europe, the FDA (Food and Drug Administration) in the USA, the PMDA (Pharmaceuticals and Medical Devices Agency) in Japan, as well as the DCGI (Drugs Controller General of India) in India. Their primary responsibility is to respond to queries, ensure approvals are granted smoothly, and submit dossiers.
-
RA Professional’s Role
RA professionals manage the Medical products lifecycle by ensuring compliance with the Regional as well as International Regulatory Laws and regulations. These professionals guide the submission and preparation of Documentation required for the authorization of Clinical Trials, marketing approval, and post-marketing surveillance in accordance with Regulatory requirements.
-
Why It Matters?
- Company reputation: Regulatory non-compliance often results in product recalls or financial penalties for a company.
- Patient access: RA professionals ensure life-saving therapies are provided to the patients at a faster rate. By integrating compliance expertise with clinical insights, CDM, Regulatory Affairs & Chemistry accelerate safe and timely patient access to breakthrough treatments.
- Global approvals: RA professionals enable global market entry through harmonized Regulatory Documentation such as CTD (Common Technical Document) or eCTD (electronic CTD).
-
Trends in RA
- Usage of Regulatory Intelligence AI tools for predicting drug or compound approval success.
- Digital submissions as well as automation (eCTD 4.0).
- Strategic focus on Regulatory standards early in R&D (Research and Development).
- Deep Insights on RA
-
- Global Regulatory Harmonization: Since multinational Clinical Trials is becoming the new norm, RA professionals should align their submissions across authorities that adopt harmonized formats such as eCTD.
- Digital Submissions: eCTD 4.0 has recently been highlighted, and RA professionals are eager to transition to fully digital and modular submissions to enhance efficiency and compliance with Regulations.
- Regulatory Intelligence: AI-powered Tools aid RA professionals in designing more innovative strategies, anticipating queries, as well as tracking regulatory trends for a faster drug or product approval process.
RA professionals are global strategists who ensure the smoother, compliant, and faster entry of drugs or products into international markets. They also maintain a strong emphasis on Ethical governance and patient safety globally.
03 Clinical Chemistry
Clinical Chemistry professionals provide the Scientific evidence needed to evaluate a Drug or product’s Biological impact and effects.
-
What is it?
Clinical Chemistry professionals analyze bodily fluids, such as plasma, urine, and blood, for monitoring and detecting diseases, assessing patient health, and evaluating the actions of drugs. In clinical trials, it is essential to consider the following:
-
- Pharmacokinetics, i.e., how a drug travels through,
- Pharmacodynamics, i.e., effects of the drug
- Biomarker analysis, i.e., assessment of treatment effectiveness or ADR (Adverse Drug Reactions).
- Clinical Chemistry Professional’s Role
These professionals focus on analyzing Biological fluids to detect toxicity, assess the effectiveness of therapy, and monitor the action and reaction of the drug. They are essential professionals in both Drug safety evaluation and clinical trial design.
As the world moves towards more personalized and customized Therapeutics, the importance of Clinical Chemists is becoming increasingly critical.
-
Why It Matters?
- Personalized Medicine: These professionals help stratify patients for targeted therapeutics and treatments. Together with data-driven decision-making and regulatory alignment, CDM, Regulatory Affairs & Chemistry make personalized medicine safer and more effective.
- Safety profiling: They work on detecting early signs of organ toxicity in people.
- Efficacy validation: They confirm and validate the therapeutic effects of drugs through Biological changes.
-
Current Trends in Clinical Chemistry
- Point-of-care diagnostics for real-time treatment monitoring.
- Role in multi-omics such as Metabolomics, Proteomics, and Genomics for precision drug responses.
- Nano-biosensor and Lab-on-chip technologies.
-
Deep Insights into Clinical Chemists
- Biomarker Validation: Biomarkers are utilized for monitoring treatment response, predicting drug side effects, and making diagnoses.
- Omics Integration: This domain is increasingly combining Proteomics, Metabolomics, and Genomics to gain multidimensional insights. This is driving the global shift from generic therapeutics to precision and personalized treatments.
- Pharmacokinetic & Pharmacodynamic (PK/PD) Studies: They have a huge role in understanding how a drug behaves in the human body, determining optimal dosages, and evaluating time-course effects.
Clinical Chemists ensure that the drugs are scientifically sound, safe, and effective for real-world application, as well as are enablers of Precision and Personalized Medicine.
The Synergistic Triad
Although CDM, Regulatory Affairs & Chemistry are distinct domains, they are intricately connected in purpose and impact.
- The RA professional translates the validated Data into compliant and actionable document submissions of the products.
- CDM ensures the data collected is analyzable, accurate, and clean.
- Clinical Chemistry professionals provide Clinical parameters as well as raw data to be analyzed further.
This powerful triad supports a safer, efficient, and faster Drug Development process, especially essential for vaccine development, orphan drug discovery, as well as global health emergencies.
Career Opportunities in this Triad
The Life Sciences sector is growing rapidly and becoming more regulation-heavy and tech-integrated, so professionals skilled in Clinical Chemistry, RA, and CDM are in high demand. Top Pharmaceuticals, Biotechnology startups, Digital health platforms, as well as CROs (Contract Research Organizations) seek professionals skilled in these domains.
Some major career roles are listed below;
- Regulatory Affairs Strategist: These professionals lead global Regulatory planning, from early drug development through to post-market surveillance of drugs and products. They ensure smooth navigation through various Regulatory regulations with a significant focus on accuracy, Compliance, and speed.
- Clinical Data Scientist: They combine Biostatistics, Domain expertise, as well as Data Analytics to extract essential and meaningful information from complicated Clinical Data. They work on adaptive trials, AI-driven research models, as well as real-time monitoring of drugs.
- Regulatory Submissions Lead: They manage the development and submission of high-quality Regulatory Dossiers, including eCTD, IND (Investigational New Drug), BLA (Biologics License Application), and NDA (New Drug Application). They work in collaboration with Regulatory authorities, including the FDA, DCGI, PMDA, and EMA.
- Clinical Chemistry Analyst: They specialize in PK/PD profiling, laboratory diagnostics, as well as Biomarker validation. They support patient monitoring and trial design, which is a major for early toxicity detection and Precision Medicine.
- Biomarker Data Analyst: They analyze Molecular Biomarkers and Clinical data to predict drug response, support precision medicine in diagnostics and trials, as well as enable patient stratification.
- Real-World Data Manager: They primarily focus on the validation and integration of data from EHRs (Electronic Health Records), patient applications, and digital Health wearables. The work on post-marketing studies, value-based health models, as well as hybrid Clinical Trials.
- Compliance and Safety Officer: Ensures Regulatory and Clinical Compliance, identifies risk signals, supports Pharmacovigilance functions, and manages adverse Drug events.
- Omics Data Integrator: They bridge Systems Biology and Clinical Chemistry by interpreting Metabolomic, Proteomic, as well as Genomic data, which are essential for companion Diagnostics and Biomarker-based Therapies.
- Pharmacovigilance-CDM Integrator: They bridge Safety Systems and Clinical Trial data to ensure Regulatory Compliance, Pharmacovigilance throughout the drug development lifecycle, as well as real-time adverse event tracking and analyses.
In a world where Personalized Therapeutics, global compliance, and digital health are booming, these Triad career roles are growing in demand across various sectors. Skilled professionals who possess knowledge across multiple cross-functional domains are regarded as innovation catalysts, aiding in bridging communication between Technical, Regulatory, and Scientific teams.
Future Outlook
Drug Discovery’s future is becoming more about developing and manufacturing drugs as well as products that are safe, Ethical, and effective for the patients. Whether you’re a student exploring career paths or a mid-career professional looking to upskill, the CDM–RA–Clinical Chemistry triad offers resilient, high-impact, and future-forward opportunities.
With the rise of Personalized Medicine, AI-driven drug pipelines, as well as digital developments, the rulebook of the Life Sciences and Healthcare industry is evolving. Trained professionals will work at the forefront of healthcare innovation.
This triad ensures that promising drugs or products don’t remain on paper, but become real therapies that improve lives globally.
For professionals and students seeking a future-proof, high-impact career in Life Sciences, mastering this triad is a step toward shaping the future of the Healthcare sector.
The future of Drug Discovery doesn’t lie in one innovation or one laboratory, but in the collaboration of CDM, Regulatory Affairs & Chemistry, diagnostics, and data-driven regulation.
And this triad is leading the way, and so will you.