How to Land Top-Paying Pharma Regulatory Affairs Jobs with the Right Training
Every Medication has its own unique story. We’re going to explore a story that doesn’t start in the laboratory, but in the hands of Regulatory Affairs professionals. Well, shocked? They transform Scientific innovations into accessible and safe treatments for the world.
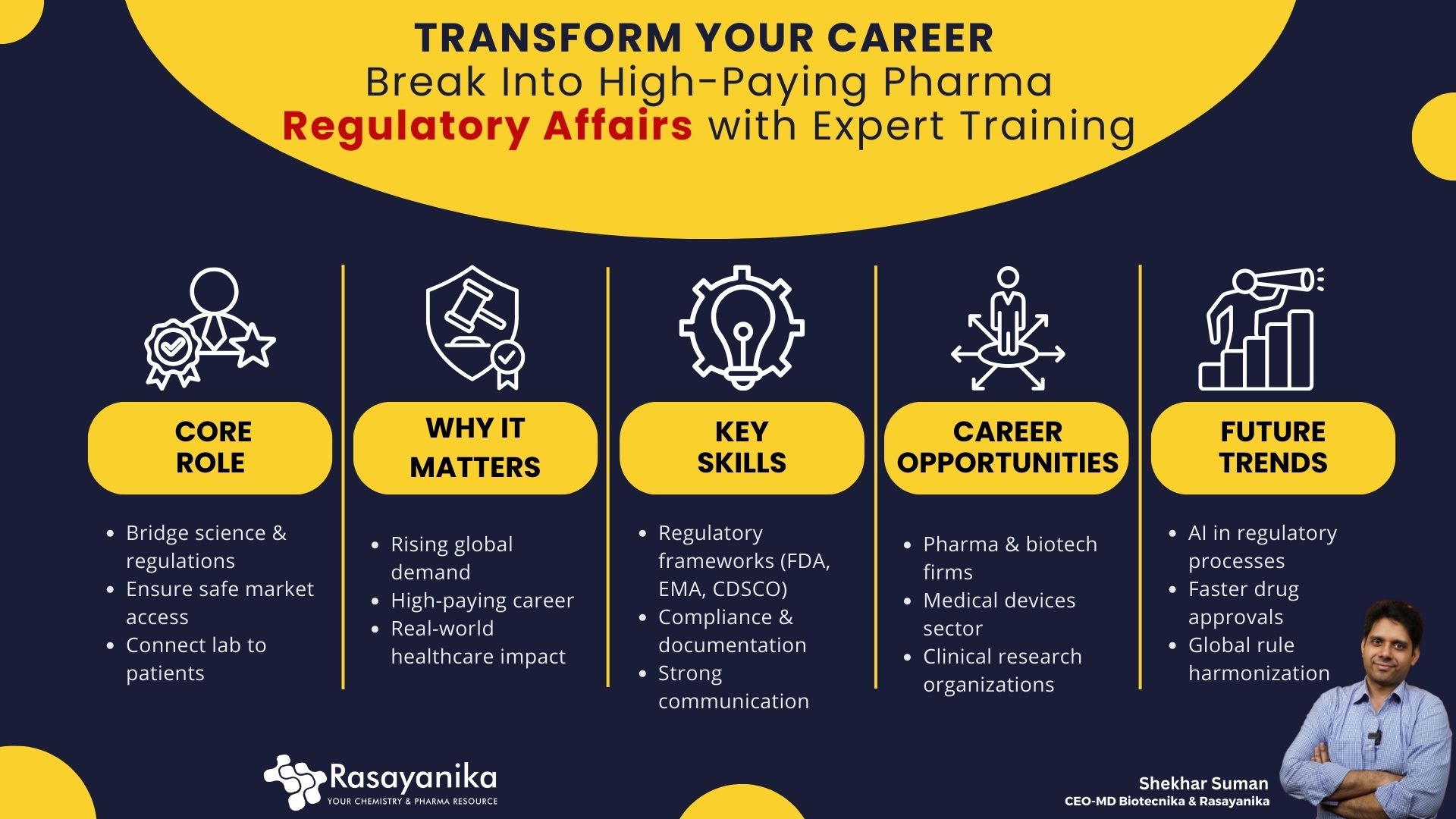
Behind each life-saving medicine, every vaccine, and every innovative therapeutic, RA professionals come into action. They bridge the gap between real-world impact and Scientific discoveries, while ensuring that laboratory innovations become accessible and safe treatments that would change and heal lives worldwide.
The Pharmaceutical industry is currently growing at a rapid rate, and the demand for skilled Regulatory professionals is high worldwide. It makes a Regulatory Affairs career highly impactful, future-proof, as well as a purpose-driven path offering high regard, competitive rewards, and opportunities worldwide.
But there’s a tiny truth that just securing a degree in Regulatory Affairs won’t set you apart from the world full of Regulatory Affairs professionals. So, to truly stand out in the crowd, aspiring Regulatory Affairs professionals need specialized training and skills. It encompasses global Regulatory knowledge, industry exposure, as well as practical skills. This would result in gaining global Regulatory opportunities , making a real impact, as well as landing top-paying Pharma Regulatory Affairs Jobs.
As Biotechnology, Medical Device, Drug Discovery, and Pharmaceutical innovation evolve, so does the demand for skilled Regulatory Affairs professionals. They manage Clinical Trial approvals, facilitate market access across multiple regions, as well as ensure drug safety. It is a highly interesting and in-demand career, as it stands at the intersection of Law, Business, and Science.
With proper preparation and a strong mindset, you can step into a future that offers prestige, power, as well as purpose to make a difference in the Global Life Sciences and Healthcare sector.
In this article, which focuses on Regulatory Affairs Career, we’ll explore the emerging industry trends, specialized training programs, as well as essential skills that would help you step into top Regulatory Affairs career roles with confidence, build an incredible future career, and make a real impact in different sectors globally.
Why Does Regulatory Affairs Matter?
The Pharmaceutical and Life Sciences industry is innovation-driven. And the Regulatory professionals ensure that every novel therapy, medical device, or drug is effective, compliant with the international Regulatory standards, and is safe to use.
Recently, the primary focus of various industries has shifted toward harmonized global regulations, patient-centric approaches, as well as faster approvals. This has created a surge in demand for Regulatory Affairs (RA) experts who can balance innovation with Global and local compliance.
Some prime responsibilities of RA include:
- Submitting and preparing Regulatory dossiers for approvals.
- Communicating between Regulatory agencies and companies.
- Monitoring safety, quality, as well as labeling of the product after commercialization.
- Ensuring Compliance with International Regulatory standards such as:
- Central Drugs Standard Control Organization (CDSCO) in India
- Food and Drug Administration (FDA) in The USA
- European Medicines Agency (EMA) in Europe
- Medicines and Healthcare products Regulatory Agency (MHRA) in the UK
- Pharmaceuticals and Medical Devices Agency (PMDA) in Japan
This career role is both strategic and technical, requiring a deep knowledge of Regulations and Science.
RA professionals manage submissions to Regulatory authorities such as the EMA (Europe), FDA (USA), PMDA (Japan), as well as CDSCO (India), and ensure global market readiness. Without these skilled professionals, even the most innovative and futuristic therapies may never reach the patients in need worldwide.
According to Grand View Research reports, the global RA market in Pharmaceuticals is projected to reach around USD 14.34 billion by the year 2030. It is growing steadily at a CAGR (Compound Annual Growth Rate) of 7.17 percentage from the years of 2025 to 2030. This highlights a rising demand for skilled RA professionals who can manage global Regulated product submissions, ensure faster approvals of therapeutics, as well as understand the evolving compliance and Regulatory requirements.
Skills for a Global RA Career Success
Success in an RA career requires multidisciplinary skills; candidates become highly attractive for Regulatory Affairs jobs worldwide. Some of the most in-demand skill sets are:
- Regulatory Intelligence: Keeping pace with evolving global guidelines, including the GxP (Good Practice), ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use), as well as the ISO (International Organization for Standardization) standards. Professionals having a knowledge of Clinical Trial Regulations, Quality standards, as well as international Regulatory guidelines are preferred.
- Scientific Knowledge: A profound understanding of Pharmaceuticals, Clinical Research, Biotechnology, as well as Life Sciences, is essential.
- Analytical Thinking: The capability to interpret Regulations and apply them in real-world scenarios for product analyses.
- Communication and Negotiation: The ability to liaise effectively with stakeholders, R&D (Research & Development) teams, as well as Regulatory authorities.
- Documentation and Compliance: Expertise in NDAs (New Drug Applications), preparing dossiers, INDs (Investigational New Drugs), device registrations, as well as CTD/eCTD (Common Technical Document and electronic Common Technical Document) submissions.
- Adaptability: Staying up to date with the new Technologies such as Digital Therapeutics and AI-driven Drug Discovery, as well as evolving Regulations worldwide.
- Strategic Thinking – Guiding product labeling, lifecycle management, global market entry, as well as risk-benefit analyses.
Regulatory Affairs Career Opportunities Globally
RA professionals work across various sectors such as Medical Devices, Pharmaceuticals, Cosmetics, Biologics, Biotechnology, as well as Nutraceuticals companies worldwide. Some high-in-demand RA career roles include:
- Clinical Regulatory Coordinator
- Regulatory Affairs Specialist/Associate
- Global Submission Manager
- CMC (Chemistry, Manufacturing & Controls) Expert
- Quality & Compliance Officer
- Regulatory Consultant/Strategist
This opens up opportunities in:
- Medical Devices & Diagnostics Companies: They ensure compliance and safety of innovative, futuristic Health Technologies.
- Consulting Companies: They advise firms on compliance frameworks as well as global product market entry strategies.
- Biotechnology & Pharmaceutical Companies: They lead Regulatory submissions for new Biologics, vaccines, and drugs.
- Government Agencies: They contribute to public health by approving and reviewing novel therapeutics.
- CROs (Contract Research Organizations): They manage Regulatory documents for Clinical Trials across countries worldwide.
As global harmonization efforts, including digital submissions and ICH guidelines, are becoming the current norm, skilled RA professionals are well-positioned for incredible Pharma jobs across Europe, the US, Asia-Pacific, and the Middle East, including top Pharma Regulatory Affairs Jobs in multinational industrial organizations. Salaries are highly competitive and rewarding, with senior RA experts generally earning six-figure packages with their experience.
The Future of Regulatory Affairs
The field of RA is evolving rapidly globally, and some major trends driving this evolution are:
- Green & Sustainability Regulations: Growing emphasis on eco-friendly drug development, packaging, and compliance.
- Global Regulatory Harmonization – Countries are aligning regulations, reducing duplication, and accelerating approvals for innovative therapies.
- AI & Digital Transformation – Artificial Intelligence and digital tools are streamlining Regulatory submissions, automating document reviews, and enabling predictive compliance.
- Cell & Gene Therapy Regulations – Specialized frameworks are being developed to handle complex Biologics, Regenerative Medicine, and Personalized Treatments.
- Patient-Centric Approaches – Regulatory frameworks are increasingly incorporating real-world evidence and patient-reported outcomes.
These advancements ensure that Regulatory Affairs will continue to be an intellectually challenging and future-proof career.
High-Paying Pharma Regulatory Affairs Jobs
Looking ahead, Regulatory Affairs will continue to be at the core of medical device development, healthcare innovations, as well as drug discovery worldwide. As industries embrace global harmonization, patient-first regulations, as well as AI-driven submissions, RA professionals will be among the most sought-after experts.
How to Get Started?
One of the biggest challenges for entry-level and mid-level professionals is gaining hands-on experience in Regulatory documentation and real-world project exposure. This is where specialized programs can bridge the gap between academic knowledge and industry readiness, helping learners step confidently into high-demand Pharma Regulatory Affairs Jobs.
The Global Regulatory Affairs Hands-on Training Program with Project Work by BioTecNika is designed to go far beyond traditional learning. What truly distinguishes this program is its powerful combination of live, interactive classes led by industry experts, intensive real-world project work, international Regulatory case studies, and dedicated placement support.
The outcome is a learning experience that not only builds knowledge but also sharpens industry readiness, confidence, and employability, enabling participants to stand out in today’s highly competitive Life Sciences job market.
How to Succeed in Landing Top-Paying RA Jobs
Entering into the Pharmaceutical Regulatory Affairs sector and securing high-paying roles requires a focused and strategic approach.
- Build a Foundation in Regulatory Frameworks: Start by gaining an in-depth understanding of CDSCO, FDA, ICH, and EMA guidelines. Top employers value candidates who can effectively apply and interpret these international regulations, which is key to securing leading Pharma Regulatory Affairs Jobs since Compliance and Regulations drive global market success and drug approvals.
- Get Trained: Hands-on training like understanding RA submission processes, working on real-world RA projects, as well as preparing Regulatory dossiers, would set you apart from the rest. This enhances the chances of landing high-paying Pharma Regulatory Affairs Jobs.
- Leverage Placement Support and Industry Networks: According to recent industry reports, entry-level Regulatory professionals in India can earn ₹4–6 LPA, while experienced professionals move into the ₹12–20 LPA range and beyond. Globally, Regulatory managers often cross $100,000 annually. Training programs that offer 100% placement assistance and mentorship help you connect with these opportunities more quickly, ensuring your skills translate into financial growth. This enables you to get a job and negotiate a competitive salary, even for fresher professionals entering the field of RA.
As the Pharmaceutical and Life Sciences industry continues to expand, Regulatory Affairs is emerging as one of the most future-proof and rewarding career paths. For aspiring professionals, this field offers not only stability but also the opportunity to secure a career in Pharma Regulatory Affairs. One can secure top Regulatory Affairs jobs, explore global Pharma jobs, as well as achieve excellent salary packages. This, in turn, makes a real impact on public health, patient safety, and global compliance standards.
However, to truly excel, one must go beyond theoretical knowledge and gain hands-on exposure, industry-relevant skills, and global Regulatory Affairs insights. This is where programs like BioTecNika’s Global Regulatory Affairs Hands-on Training play a transformative role. By combining expert-led interactive sessions, practical project experience, international case studies, and dedicated placement assistance, it empowers learners to confidently step into the workforce with an edge over their peers and excel in global Pharma Regulatory Affairs Jobs.