Think of yourself uncovering harmful quality drugs, and preventing them from reaching the Pharmacy or a patient. Imagine possessing the ultimate power of Law and Science to shut down unauthorised and illegal laboratories, thereby protecting millions of lives. This is not a scene from a crime thriller movie, but is the incredible reality of a Drug Inspector worldwide, or even in India. These unsung heroes watch the line of defense between dangerous counterfeits and good quality drugs, eventually protecting our Healthcare system. While the doctors prescribe and the Pharmacists dispense medicines, the Drug Inspector ensures that the drugs are effective, safe, as well as genuine. With increased importance to drug safety and an increased rise in Pharmaceutical crimes, being a Drug Inspector won’t be just a random job, but would be a mission!
Do you know who the world’s third-largest producer of Pharmaceuticals is? It’s India, supplying over 50 percent of the total vaccine demand globally. And further, do you know who’s at the heart of this huge industry? They are the Drug Inspectors!
The Indian pharmaceutical industry has grown into a global leader. This expansion required stringent regulation and actions to ensure drug safety, compliance, and quality.
Well, if you aspire to grow or start a career as a Drug Inspector, then this article will provide you with an incredible roadmap, shaping your career!
The Role of a Drug Inspector
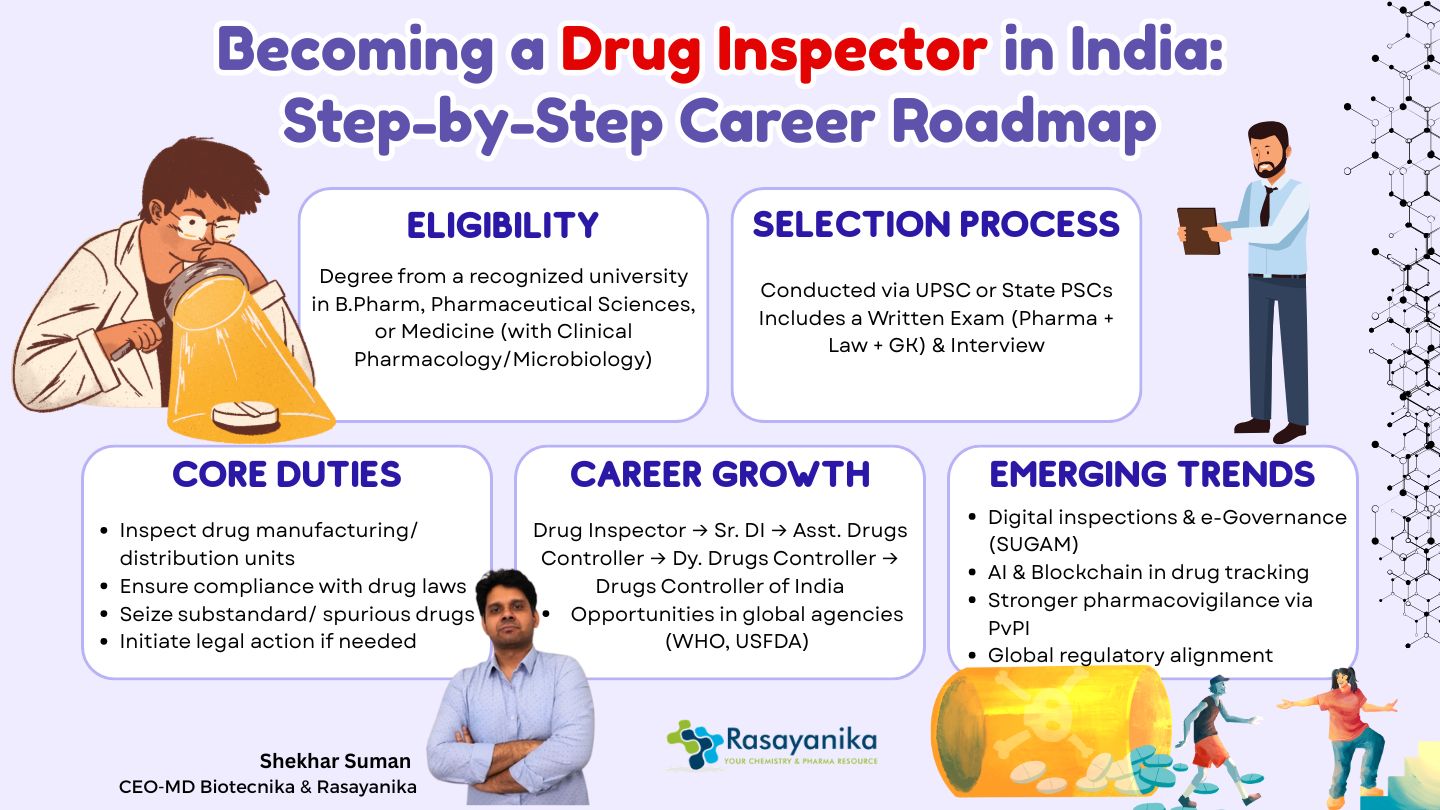
A Drug Inspector is an official (government-appointed) who is responsible for enforcing regulations as well as monitoring drug development. They ensure compliance related to drug and cosmetic distribution, sale, as well as manufacturing, as per the Drugs and Cosmetics Act (1940). They are appointed either by the Central or State Governments, and they inspect drug manufacturing facilities, ensuring compliance with Good Manufacturing Practices (GMP). Drug Inspectors have an important role in detecting spurious or substandard drugs and maintaining high drug quality in the domestic as well as global Pharmaceutical market.
Key Responsibilities Include:
- Verify adherence to GMP
- Conduct rigorous inspections of Drug retail outlets and Drug manufacturing facilities.
- Investigate consumer complaints as well as identify any violations
- Collecting Drug samples for Qualitative and Quantitative laboratory testing
- Prepare legal reports
- Coordinate with enforcement agencies and drug testing laboratories
With issues such as fake drugs as well as raising demand for Drug transparency, the responsibilities of Drug Inspectors grows and expand, emphasizing their importance in the Pharmaceutical and Healthcare ecosystem.
These are a few steps in becoming a successful Drug Inspector in India, as follows:
Step 01 – Educational Qualifications
For an aspirant to qualify for the position of a Drug Inspector, the candidates should possess an official degree from a reputed University or College in one of the following fields:
- Degree in Pharmaceutical Sciences
- Degree in Medicines (with specialization in Microbiology, Clinical Pharmacology, or other related fields)
- B.Pharm (Bachelor of Pharmacy) degree
The Degrees should be recognized by the “Pharmacy Council of India” to ensure successful eligibility. Expertise in subjects such as Pharmaceutical Chemistry, Drug Laws, as well as Pharmacology can offer an added advantage during competitive examinations.
The qualifications for Drug Inspector posts mainly require a Pharmaceutical Sciences or a B.Pharm degree.
Certifications and courses in Clinical Research, Pharmaceutical Quality Control, or Regulatory Affairs can give you an upper hand in the recruitment process.
Step 02 – Relevant Industry Experience
Having 1 to 2 years of relevant experience in Quality Assurance, Regulatory Compliance, or Pharmaceutical Manufacturing can strengthen your candidature. Good experience enhances your practical insights into inspection protocols and industry operations. Such valuable experiences are highly valued and recognised by some PSCs (State Public Service Commissions). Central bodies such as UPSC (Union Public Service Commission) and some State PSCs prefer candidates with at least 18 months of working experience in drug testing or manufacturing.
Experience areas may include:
- Exposure to GMP compliance
- Involvement in drug production as well as documentation
- QA (Quality Assurance) or QC (Quality Control) in Pharmaceutical companies
Step 03 – Competitive Examinations
Recruitment for Drug Inspectors is mainly conducted through competitive written exams organized by the UPSC or the respective State PSCs, such as TNPSC, WBPSC, and MPSC.
Exam Pattern:
- Written Test: Objective questions from subjects like Pharmacology, Pharmaceutical Chemistry, Toxicology, Drug Laws, Microbiology, GMP, as well as Ethics.
- Interview: Shortlisted candidates are further interviewed to assess their ethical judgment, practical readiness, and technical knowledge.
Preparation Tips:
- Practice the last 5-10 as well as previous years’ question papers thoroughly and regularly.
- Stay updated on the recent Pharmaceutical news and Regulatory developments.
- Study standard references like Lachman’s Theory and Practice of Industrial Pharmacy and Remington’s Science and Practice of Pharmacy.
Step 04 – The Interview Stage
The interviews assess your technical knowledge as well as your professional aptitude and skill sets. The candidate should expect scenario-based questions that evaluate their knowledge of Ethical standards, understanding of drug regulations, as well as decision-making and other soft skills.
Preparing with mock interviews as well as staying well-informed about current Drug Regulatory aspects can significantly improve the candidature and performance.
Step 05 – Appointment & Comprehensive Training
Selected candidates are appointed by the CDSCO (Central Drugs Standard Control Organization) or the State Drug Control Departments. Initial Drug Inspector training is conducted to impart Legal and practical knowledge, which includes practical hands-on sessions like sample collection procedures, mock raids, as well as field inspections
The Training Includes:
- SOPs (Standard operating procedures) for Drug inspection and sampling.
- Understanding Pharmaceutical Standards and Drug Laws.
- Documentation and Reporting protocols.
- Overview of International Regulatory practices including the FDA (Food and Drug Administration), WHO-GMP (World Health Organization – Good Manufacturing Practices), and EMA (European Medicines Agency).
- Coordination with Legal authorities and laboratories.
Drug Inspectors hold under “Section 21” of the Drugs and Cosmetics Act, 1940, regarding inspection, seizure, and entry powers, which underpins their official duties. The training ensures that the officers are prepared to meet the national as well as the global Compliance Standards effectively and successfully.
Career Opportunities
- A career as a Drug Inspector offers a clear progression path, which is as follows:
√ Senior Drug Inspector
√ Assistant Drugs Controller
√ Deputy Drugs Controller
√ Drugs Controller
√ DCGI (Drugs Controller General of India)
- Opportunities to work with International bodies such as USFDA, WHO (World Health Organization), or private regulatory consulting firms.
Beyond Government roles, experienced Drug Inspectors can further explore careers in Healthcare organizations such as the WHO, Regulatory Affairs with leading Pharmaceutical companies, as well as Consultancy roles focusing on Pharmaceutical Policies.
With increasing growth in Technology and Digitization adoption in Pharmaceutical regulation, Drug Inspectors’ familiarity with AI-enabled audits and digital tools is highly valued. These professionals ensure Compliance with International benchmarks and safeguard public trust in drugs, as well as serve as the backbone of National Drug Safety Programs.
Regulatory Updates & Emerging Technologies
The Regulatory landscape in India is rapidly evolving, reflecting the Government’s commitment to safe, efficient, and fast public Health responses. Furthermore, India’s increasing alignment with ICH (International Council for Harmonisation) guidelines ensures that the Pharmaceutical compounds meet the global Regulatory Standards.
Technological innovation is also reshaping the Drug Inspector’s toolkit. Artificial Intelligence (AI) and Machine Learning (ML) models are being deployed to enhance Drug Risk Assessment as well as inspection accuracy. For example, AI-driven drug audits can analyze manufacturing data patterns to identify anomalies before physical inspections occur. Furthermore, Blockchain Technology is emerging as a solution for Pharmaceutical traceability, which aids Drug Inspectors to reduce counterfeit/fake drug risks as well as verify Drug authenticity effectively.
These futuristic developments equip Drug Inspectors with advanced tools and enhance their positions in contributions to India’s growing stature in the global Pharmaceuticals market.
- Digital Inspections: Use of online portals such as e-Governance tools and SUGAM (System for Unmanned Gateways Approval of Manufacturers).
- AI and Blockchain: Potential advanced tools for tracking the drug supply chain.
- Pharmacovigilance Collaboration: Integration with Pharmacovigilance Programme of India (PvPI) to track adverse drug reactions (ADRs).
- Global Alignment: Increased alignment with WHO-GMP, EMA, as well as USFDA standards.
A career as a Drug Inspector provides a chance to serve the nation by ensuring Public safety as well as Public Health. This career demands knowledge, a commitment to public safety, and dedication. If you’re inspired to make a tangible difference in Regulatory Affairs and Healthcare, begin by exploring recognized preparatory courses in GMP compliance, Regulatory Affairs, as well as Pharmacy Law. Regularly check official notifications from the UPSC and the State PSCs to stay updated on examination requirements and schedules.
For those passionate about Pharmaceuticals,Regulatory Affairs, and Law, then this career path presents both challenges and rewards. With dedication and preparation, you can contribute to building a safer, healthier society.
Investing time in understanding recent regulatory changes and technological advancements is crucial. This knowledge will give you a competitive edge. Take the first step now—prepare with trusted resources, stay informed, and position yourself as a future guardian of drug safety in India’s Pharmaceutical landscape. Embark on Your Journey as a Drug Inspector Today!















































